Impact of the polymer dispersity on the properties of curcumin/polyvinylpyrrolidone amorphous solid dispersions
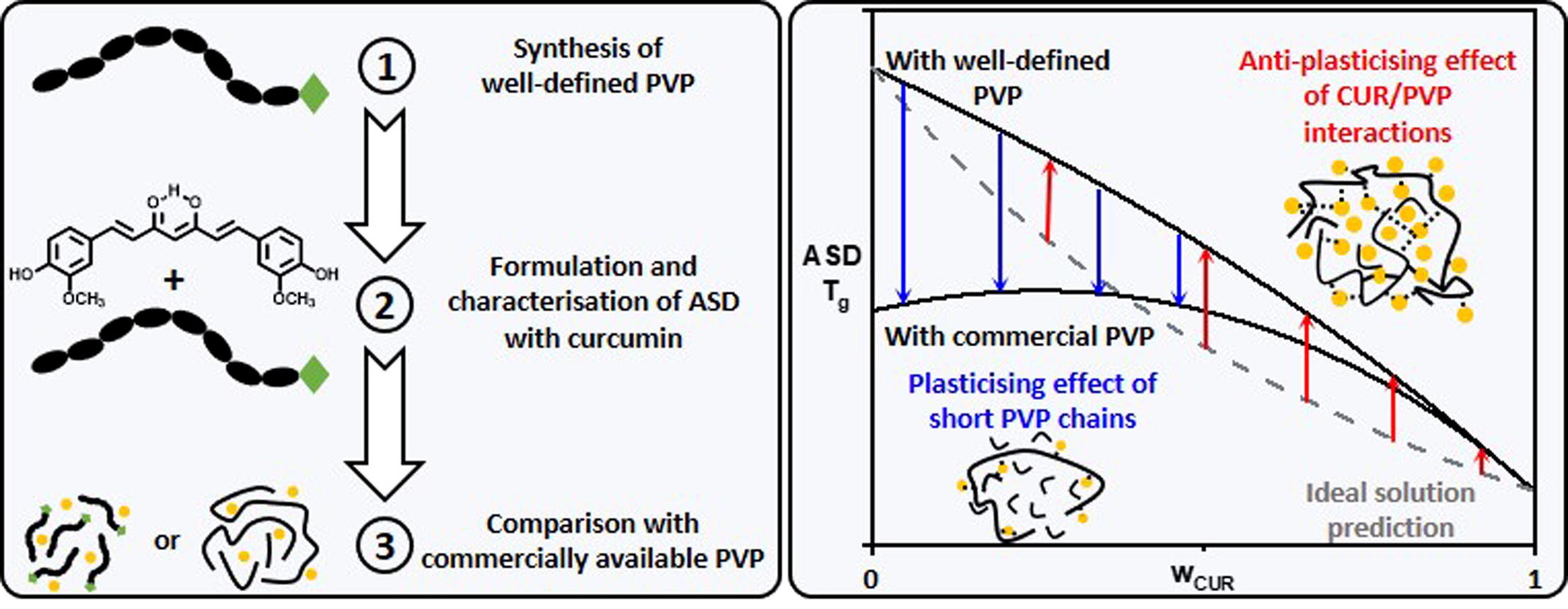
Amorphous solid dispersions (ASD) are known to enhance the absorption of poorly water-soluble drugs. In this work we synthesise well-defined Polyvinylpyrrolidone (PVP) to establish the impact of dispersity and chain-end functionality on the physical properties of Curcumin (CUR)/PVP ASD. Thermodynamic characterisation of synthesised PVP emphasises a strong effect of the dispersity on the glass transition temperature (Tg), 50 °C higher for synthesised PVP than for commercial PVP K12 of same molar mass. This increase of Tg affects the thermodynamic properties of CUR/PVP ASD successfully formulated up to 70 wt% of CUR by milling or solvent evaporation.
Highlights
- Sur-saturated Curcumin/PVP amorphous solid dispersions (ASD) obtained by milling.
- PVP low dispersity drastically increases glass transition temperature of ASD with Curcumin.
- Shortest PVP chains reduce the anti-plasticisation effect of Curcumin-PVP interactions.
- Solubility of Curcumin is increased in ASD formulated with well-defined PVP.
The evolution of both the Tg and CUR solubility values versus CUR content points out the development of fairly strong CUR-PVP interactions that strengthen the antiplasticising effect of PVP on the Tg of ASD. However, for ASD formulated with commercial PVP this effect is counterbalanced at low CUR content by a plasticising effect due to the shortest PVP chains. Moreover, the overlay of the phase and state diagrams highlights the strong impact of the polymer dispersity on the stability of CUR/PVP ASD. ASD formulated with low dispersity PVP are stable on larger temperature and concentration ranges than those formulated with PVP K12.
Read more here
Materials
N-vinylpyrrolidone (NVP) (TCI; 97 %) was distilled under reduced pressure. 2,2′-azobis(isobutyronitrile) (AIBN) (Sigma Aldrich; >99 %) was purified by recrystallisation from methanol before use. Curcumin (CAS n° 458–37-7, purity 97 %) was purchased from TCI while PVP K12 ( 2,000–3,000 g·mol−1), PVP K17 (7,000–11,000 g·mol−1), PVP K25 (28,000–34,000 g·mol−1), PVP K30 ( 44,000–54,000 g·mol−1), PVP K90 ( 900,000–1,200,000 g·mol−1) were purchased from BASF.
Simon Samsoen, Émeline Dudognon, Gaëlle Le Fer, David Fournier, Patrice Woisel, Frédéric Affouard, Impact of the polymer dispersity on the properties of curcumin/polyvinylpyrrolidone amorphous solid dispersions, International Journal of Pharmaceutics, 2024, 123895, ISSN 0378-5173, https://doi.org/10.1016/j.ijpharm.2024.123895.

