Formulation and in vitro evaluation of pramipexole orally disintegrating tablets for pediatric restless leg syndrome
Abstract
In this study, orally disintegrating tablets (ODT) of pramipexole dihydrochloride monohydrate (PPX) was developed with direct compression method by using ready-to-use excipients Parteck® ODT, Pharmaburst® 500, Ludiflash®, F Melt®, and Prosolv® Easytab SP for pediatric restless leg syndrome (RLS). The formulated ODTs were circular in shape with a total weight of around 100 mg, which was appropriate for pediatric use. In spite of very low content of the drug, content uniformity could be obtained successfully in accordance to the pharmacopoeial specification with a satisfactory mechanical strength in terms of hardness and friability. However, formulations based on Parteck® ODT and Ludiflash® could not achieve a disintegration time <30 s according to in vitro disintegration test, which was also supported by the simulated wetting test. The optimal ODTs based on Pharmaburst® 500, F-Melt® and Prosolv® Easytab SP were further evaluated for in vitro dissolution study. A very fast release of the drug was observed with these formulations that reached a peak value in 10 min., which was superior than that of the reference conventional tablet formulation of PPX. As a result, pediatric orally disintegrating tablets of PPX were successfully formulated with Pharmaburst® 500, F-Melt® and Prosolv® Easytab SP by using direct compression method with suitable characteristics, which can be further studied to use in pediatric RLS.
1. INTRODUCTION
Restless leg syndrome (RLS), also known as Willis-Ekbom disease, is a frequent sensory motor disorder characterized by a strong impulse to move the limbs during rest or is exacerbated by rest, and disappears during movement or is relieved after movement [1, 2]. The symptoms of RLS frequently worsen over time and generally deteriorate with aging [1-3]. Furthermore, RLS has been reported as the fourth most common cause of insomnia, particularly at the start of sleep [2]. Most of the population-based studies have reported a low prevalence in most Asian populations, which vary between 1% and 3%. However, a significantly higher prevalence has been found in Europe and North America, ranging from 5% to 13% [1].
The mean age for the onset of RLS symptoms has been reported to be 27 years [4]. Although approximately 38–45% of adults have been reported to have onset of symptoms before age 20 years, with 13% of patients reporting symptoms before the age of 10, most patients are not diagnosed for several years after their disease first manifests [2]. Since the diagnosis of RLS in childhood is complicated with defined symptoms based on previous studies, it may be more prevalent than estimated [5]. However, a prevalance of 2-4% in school-aged children and adolescents has been reported [6]. Due to difficulty sleep onset or staying asleep, children with RLS may exhibit conduct issues like aggression, inattention, hyperactivity, and daytime somnolence. These issues can cause serious affect on a child’s school performance, social development, and interactions with others, resulting in incorrect diagnoses of a variety of psychiatric conditions, including attention deficit hyperactivity disorder (ADHD), among others [5, 6].
Currently, there is no broadly approved agents available for the treatment of RLS, and the treatment choice varies depending on severity of the disease. For instance, while levodopa or a dopamine agonist may be used to treat patients with intermittent RLS, those with severe symptoms may need to be treated with potent opioids. Additionally, benzodiazepines, which are hypnotic and anxiolytic drugs, as well as other sedative medicines that may provide symptomatic relief, but have no effect on the course of RLS have traditionally been used in the treatment of RLS [7, 8]. Moreover, it was proposed that D3 dopamine receptor agonists possess neuroprotective effects by increasing the secretion of dopamine neurotrophic factor in tissue culture [9]. Pramipexole (PPX), a non-ergot D3 autoreceptor agonist, is prescribed to treat idiopathic Parkinson’s disease. Currently, it is labeled for the treatment of moderate to severe RLS [7, 8, 10, 11]. However, since PPX is available only as tablet form at various strengths which are not specified for pediatric use, there is a necessity for the more child-friendly dosage forms of PPX.
Modern medicine is dependent on patient-friendly dosage forms, which are also necessary for effective pediatric drug therapy. Experts have long suggested a paradigm shift away from traditional liquid medication forms and toward cutting-edge oral solid dosage forms for pediatric use [12, 13]. Pediatric orodispersible tablets stand out as the ideal dosage form for children among them, since they combine the benefits of oral and solid dosage forms, are well-tolerated even by newborns, and allow for personalized dosing [12-14]. Orally disintegrating tablets (ODTs) are solid pharmaceutical dosage forms that quickly dissolve in the mouth of the patient without the use of water [15-18]. It is a useful tool for patients who might have trouble taking traditional solid oral dosage forms because of a mental illness, a physical disability, a lack of access to water, or because they are too young or old. It can help to minimize the difficulty of treatment on patients and caregivers due to its easy administration. It has been demonstrated in numerous studies that patients prefer ODTs to conventional tablets [19, 20]. ODTs can be prepared by various methods as reported in the literature [15-17]. Among those, the direct compression method presents advantages such as simple technology with usually no necessary modifications to conventional compression apparatus; therefore, it is commonly utilized for the preparation of ODTs [12, 18]. ODTs prepared with this method generally possess satisfactory mechanical properties like good hardness and low friability [16]. Due to the critical effect of link between excipients and other formulation components as well as methods on these characteristics, the ready to-use excipients stands out with many beneficial attributes for the direct compression of ODTs [12, 18]. Depending on the aforementioned requirements, the aim of this study was to develop and evaluate pediatric PPX orally disintegrating tablets for restless leg syndrome in children, based on ready-to-use excipients including Parteck® ODT, Pharmaburst® 500, Ludiflash®, F-Melt®, and Prosolv® Easytab SP by utilizing the advantages of direct compression method.
2. RESULTS AND DISCUSSION
2.1. Fourier transform infrared spectroscopy study
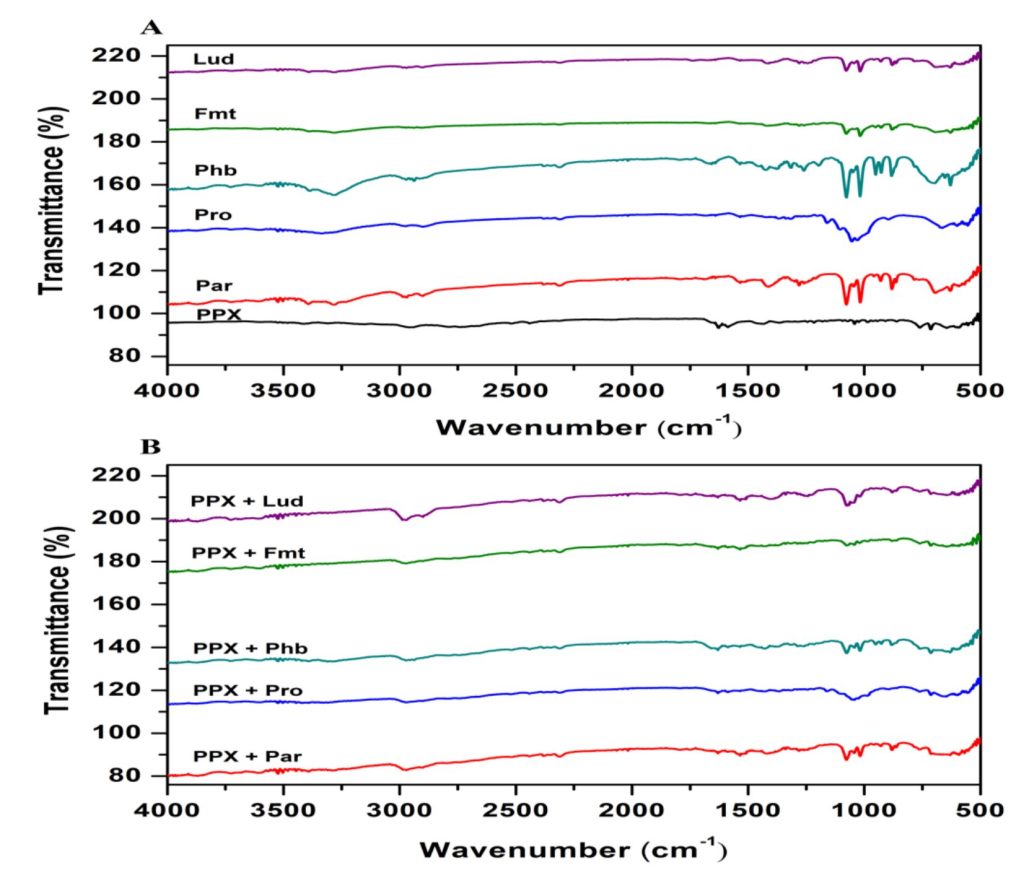
The chemical and physical incompatibility of drugs and excipients in solid state can significantly affect the critical formulation properties such as stability, dissolution rate, and bioavailability. There is not an availabile procedure that is universally used to estimate the compatibility of drugs with excipients. Thermoanalytical methods are utilized to routinely investigate and predict any physicochemical incompatibility between drugs and pharmaceutical excipients. These techniques are widely applied alone or in combination with with microscopy, spectroscopy (UV, IR), and X-ray powder diffractometry [21, 22]. Because of these reasons, FTIR analyses were conducted to examine possible drug excipient interactions before the formulation studies.
FTIR spectras of pure PPX and pure ready-to-use excipients as well as physical blends of PPX with these excipients are shown in Figures 1A and 1B, respectively. The characteristic absorption peaks of the pure PPX were obtained at the wavenumbers of 3412, 2958, 1587, 1365 and 761 cm-1 indicating, the functional groups of N-H stretching, C-H stretching, C=C stretching, C-N stretching and C-H bending, respectively (Fig 1A). All of the characteristic peaks for the pure drug were also observed in the spectrum of physical mixture (Fig 1B), indicating that the pure drug and the excipients did not interact chemically. This confirms the compatibility of PPX and the used excipients in the ODT formulations [23].
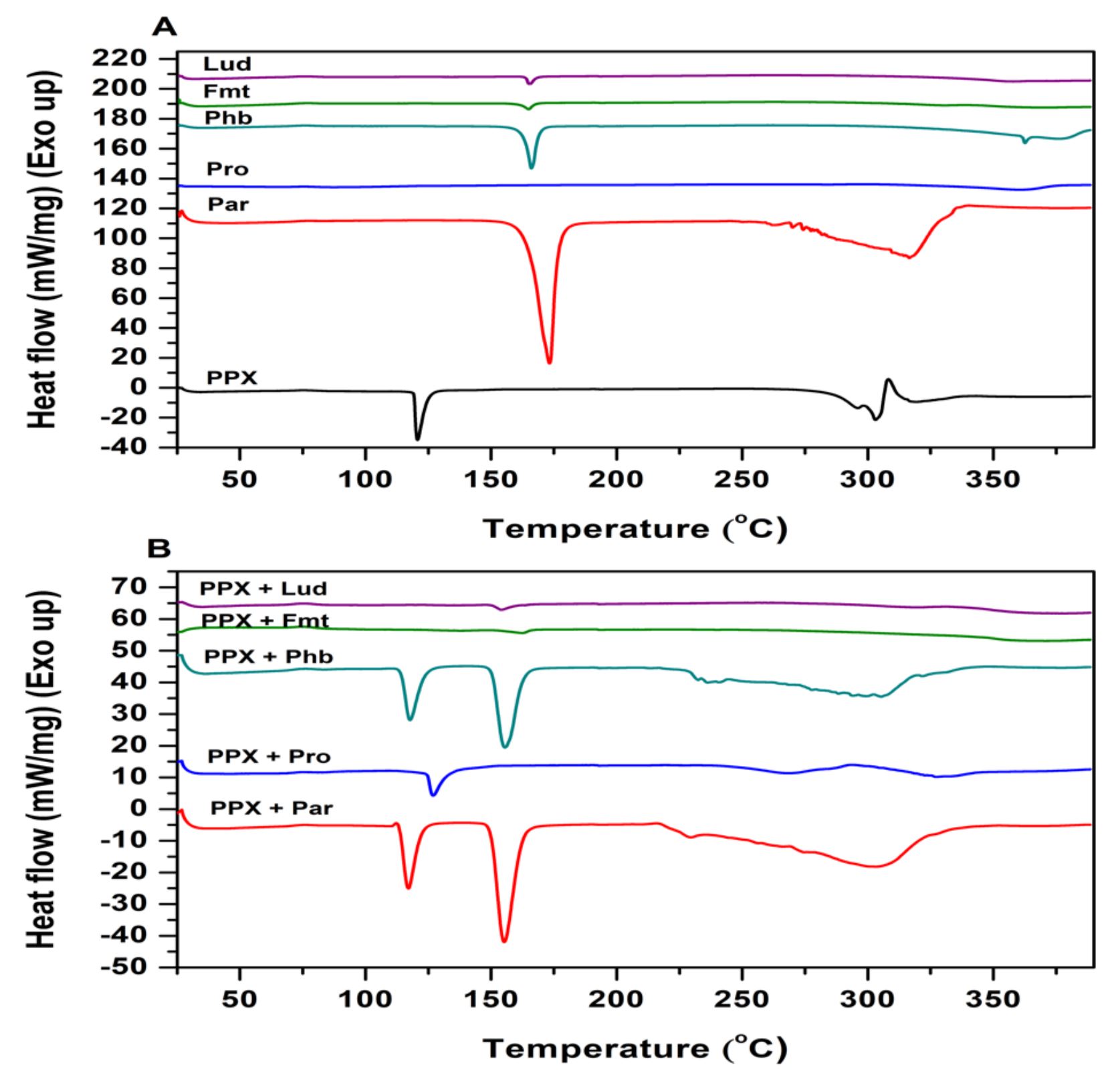
2.2. Differential scanning calorimetry study
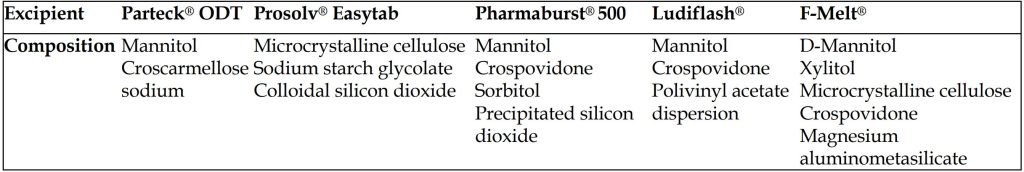
Thermal analyses are used to asess a sample characteristic while the sample is heated or cooled at a constant rate of temperature change or maintained at a fixed temperature in a specified atmosphere. For this purpose, an indispensible thermal analysis method DSC was conducted in addition to FTIR analyses to examine possible drug excipient interactions [24]. The DSC thermograms of PPX, ready-to-use excipients and binary mixtures are demonstrated in Figures 2A and 2B. The DSC curve of PPX (Figure 2A) showed a peak (endothermic event) corresponding to the dehydration of monohydrate form drug at 120.68 °C, and melting occured in the range of 291.23°C to 307.3°C [25]. The DSC curves of excipients also showed the characteristic thermal behaviour of each excipient, which is composed co-processing of powders with special purposes (Table 1). The thermogram of excipients except for Prosolv® Easytab SP which containing microcrystalline cellulose (MCC) instead of mannitol (MAN) showed an endothermic peak at around between 165 °C and 169 °C because of the MAN, which constitutes the base of the co-processed excipient (pure MAN has a melting range from 166 to 168 °C). Furthermore, Prosolv® Easytab SP shows an endothermic peak at 89.02 °C corresponding to melting point of sodium starch glycolate (SSG) in the co-processed excipient. The thermograms of drug-excipient blends did not exhibit any new peaks without slight shifts, which was a indicative for absence of incompatibility between drug and excipients. However, the slight shifts in the peaks could occur due to mixing process, as it has been reported to cause a reducing effect on the purity of the components [26].
Table 1. Composition of ready to use excipients used for formulations of pramipexole dihydrochloride monohydrate orally disintegrating tablets
See the full brochure on “Pramipexole Pediatric Restless Leg Syndrome” here
(click the picture to download the brochure)
Source: PDF “Pramipexole Pediatric Restless Leg Syndrome“
Formulation and in vitro evaluation of pramipexole orally disintegrating tablets for pediatric restless leg syndrome, Ömer TÜRKMEN, Leyla BEBA POZHARANI , Moein AMEL, Department of Pharmaceutical Technology, Faculty of Pharmacy, Van Yüzüncü Yıl University, Van, 65080, Türkiye. 2 Faculty of Pharmacy, Eastern Mediterranean University, Famagusta, North Cyprus, Mersin 10, Türkiye. Corresponding Author. Received: 06 March 2023 / Revised: 20 March 2023 / Accepted: 20 March 2023
See the webinar:
“Rational Selection of Cyclodextrins for the Solubilization of Poorly Soluble Oral Drugs”, 8. November 2023:
Get more information & register here for free:



