Investigating the prilling/vibration technique to produce gastric-directed drug delivery systems for misoprostol
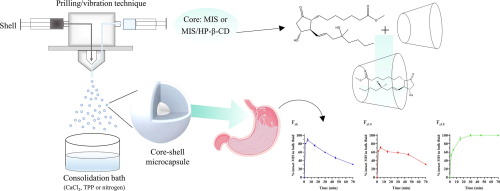
Prilling/vibration technique to produce oral microcapsules was explored to achieve local delivery of misoprostol (MIS), a prostaglandin E1 analogue indicated for the treatment of gastric-duodenal ulcers, at the gastric mucosa. To improve MIS chemical stability and reduce its associated systemic side effects, drug delivery systems were designed and developed as microcapsules consisting of a core of sunflower oil and MIS (Fs6 and Fs14) or a MIS complex with hydroxypropyl-beta-cyclodextrin (HP-β-CD) (Fs18), confirmed by specific studies, and a polymeric shell. The produced microcapsules showed high encapsulation efficiencies for those with MIS solubilized in sunflower oil (>59.86%) and for the microcapsules with MIS/HP-β-CD (97.61%).
To demonstrate the ability of these systems to deliver MIS into the stomach, swelling and drug release experiments were also conducted in simulated gastric fluid. Among the three formulations, FS18 showed gastric release within 30 minutes and was the most advantageous formulation because the presence of the MIS/HP-β-CD inclusion complex ensured a greater ability to stabilise MIS in the simulated gastric environment. In addition, these new systems have a small size (<540 µm), and good flow properties and the dose of the drug could be easily adapted using different amounts of microcapsules (flexibility), making them a passepartout for different age population groups.
Download the full article as PDF here: Investigating the prilling/vibration technique to produce gastric-directed drug delivery systems for misoprostol
or read it here
Materials
HP-β-CD (Cavasol W7, HP-β-CD with MW= 1540, molar substitution degree SD= 7), sodium alginate with a mannuronic acid to the guluronic acid ratio (M/G) of 1.8–2.2, Helianthus Annuus seed oil organic refined (density 0.9205 g/mL 25 °C), and extra virgin olea europaea oil (density= 0.920 g/mL at 25 °C) were obtained from Farmalabor Srl (Canosa di Puglia, Italy). Misoprostol (MW= 368.51), chitosan (low molecular weight MW= 50,000 – 190,000 Daltons), sodium triphosphate pentabasic, calcium chloride, and L-(+)-lactic acid were purchased from Sigma-Aldrich (Milan, Italy). Carbopol® 971P NF was purchased from Lubrizol (Bruxelles, Belgium). Amprac 01 was kindly donated by Evonik Nutrition & Care GmbH (Rofarma Italia Srl, Gaggiano, Italy). All solvents and other salts used were of analytical/technical grade and purchased from Sigma Aldrich.
Vita D’Amico, Nunzio Denora, Marianna Ivone, Rosa Maria Iacobazzi, Valentino Laquintana, Annalisa Cutrignelli, Massimo Franco, Michele Barone, Antonio Lopalco, Angela Assunta Lopedota, Investigating the prilling/vibration technique to produce gastric-directed drug delivery systems for misoprostol, International Journal of Pharmaceutics,
2024, 123762, ISSN 0378-5173, https://doi.org/10.1016/j.ijpharm.2023.123762.
Read more on “Alginates as Pharmaceutical Excipients” here:


