Is it advantageous to use quality by design (QbD) to develop nanoparticle-based dosage forms for parenteral drug administration?
Abstract
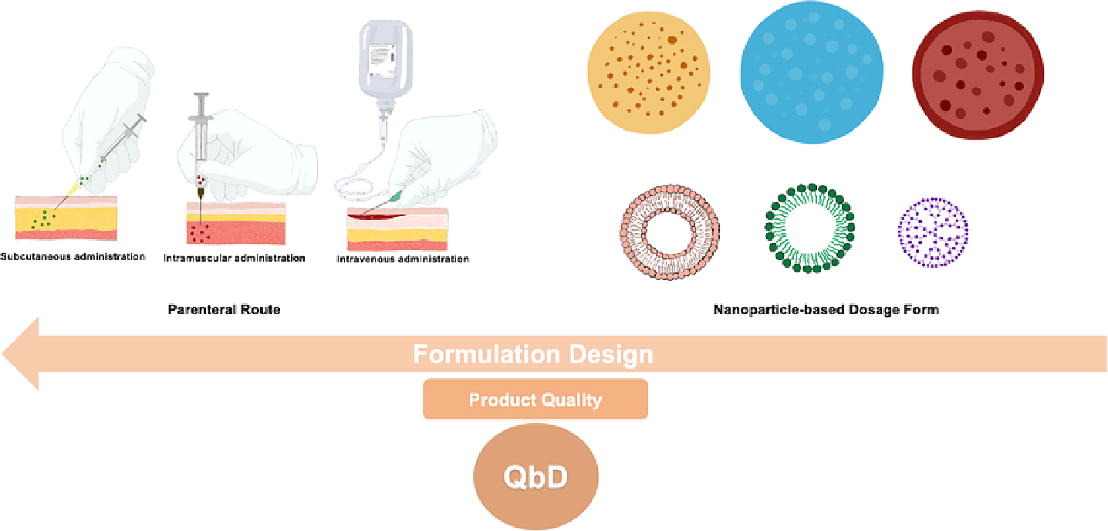
Parenteral administration is one of the most commonly used drug delivery routes for nanoparticle-based dosage forms, such as lipid-based and polymeric nanoparticles. For the treatment of various diseases, parenteral administration include intravenous, subcutaneous, and intramuscular route. In drug development phase, multiparameter strategy with a focus on drug physicochemical properties and the specificity of the administration route is required. Nanoparticle properties in terms of size and targeted delivery, among others, are able to surpass many drawbacks of conventional dosage forms, but these unique properties can be a bottleneck for approval by regulatory authorities.
Quality by Design (QbD) approach has been widely utilized in development of parenteral nanoparticle-based dosage forms. It fosters knowledge of product and process quality by involving sound scientific data and risk assessment strategies. A full and comprehensive investigation into the state of implementation and applications of the QbD approach in these complex drug products can highlight the gaps and challenges. In this review, the analysis of critical attributes and Design of Experiment (DoE) approach in different nanoparticulate systems, together with the proper utilization of Process Analytical Technology (PAT) applications are described. The essential of QbD approach for the design and development of nanoparticle-based dosage forms for delivery via parenteral routes is discussed thoroughly.
Introduction
Derived from the Greek words “para” (besides) and “enteron” (the intestine), the term parenteral can be transcribed as “outside the digestive tract”. Accordingly, it includes administration routes, which are not intestinal nor duodenal, and it usually refers to subcutaneous (SC), intramuscular (IM), or intravenous (IV) administration of drugs. When we administer the drug through the parenteral route, we have an immediate therapeutic effect that is useful in emergency situations. Some drugs can’t be administered via non-injectable routes due to their poor bioavailability, such as paclitaxel (Yerlikaya et al., 2013). Disadvantage of parenteral administration is infection risk on the administration site when the body’s natural barriers are bypassed. Further, in the case of dosing error, it is difficult to reverse the effect. Due to the stringent requirements of parenteral dosage forms, including sterility, there is a need to develop alternative delivery systems that ideally overcome the multiparametric manufacturing challenges while providing quality, efficacy, and safety (Nema and Ludwig, 2019).
Nanomedicines are the applications of nanotechnology in drug discovery and delivery. The use of nanoparticles offers several advantages, including drug stability, enhanced drug release, and drug cellular uptake. The concept was introduced by the United States government in 2000 with the creation of a new initiative called the National Nanotechnology Initiative (NNI). From the beginning, nanomedicines have focused almost exclusively on tumor-targeted drug delivery, which has many advantages (Park et al., 2022), but the applications of nanoparticle-based drug delivery systems have also faced challenges (Wang et al., 2021). Anticancer nanoformulations have been developed for intravenous use and, alternatively, for intradermal, subcutaneous, and oral use since the administration route determines the biodistribution pattern of drugs (Wang et al., 2021). Over the years, successful commercial nanoparticle-based anticancer dosage forms have been developed, including Mylotarg®, Doxil®, and Abraxane®, among others (Park et al., 2022).
The use of nanotechnology has some advantages and disadvantages in many fields and in various nanoparticle-based dosage forms. The main advantages include prevention of degradation of the loaded drug, specificity and targeted delivery, and improved bioavailability and efficiency (Adepu and Ramakrishna, 2021) (Pozharov and Minko, 2023). However, we also find some disadvantages, such as the high value of their production, the unexpected diffusion of these nanoparticle-based dosage forms into the lungs and some body barriers, which may lead to toxicity, and the special storage conditions of lipid-based nanoparticles (Adepu and Ramakrishna, 2021) (Pozharov and Minko, 2023).
Formulation design is an essential step in the development of nanoparticle-based dosage forms, and the scale-up of lab-scale preparation methods to industrial production scale is a barrier to bringing nanotherapeutics to market, and there is still a large gap between the progress made in research and in the clinic (Villa nova et al., 2015). Large amounts of financial resources are usually devoted to drug development studies. Therefore, maintaining quality attributes at an affordable cost within a short period of time has become important to both academia and the pharmaceutical industry, and consequently, the implementation of the Quality by Design (QbD) approach has received much attention (Barbalata et al., 2022). The elements and the QbD approach are detailed in the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH) guidelines, such as ICH Q8, Q9, Q10, Q11, Q12, and more recently Q13 (Grangeia et al., 2020).
The elements of QbD include the description of the Quality Target Product Profile (QTPP), in particular the ideal parameters that the product should achieve, the identification of characteristics in the formulation named Critical Quality Attributes (CQAs), Critical Material Attributes (CMAs) and Critical Process Parameters (CPPs), the results of the Risk Assessment (RA), which is the crucial step because it supports the impact of the CQAs and the CPPs on the target product profile, key points of the Design of Experiments (DoE), and the definition of Design Space (DS) (Németh et al., 2022). This approach has been proven successful in oral drug delivery systems, using different experimental designs that resulted in improved bioavailability and stability (Beg et al., 2019c).
This review aims to identify the predominant elements of QbD in nanoparticle-based parenteral drug delivery dosage forms and their importance in achieving the ideal final product. It aims to help understand and improve preparation processes and operations, accelerate pharmaceutical development, shorten the time between research and clinical applications, and propose a strategy to overcome key obstacles. At the end of the review, we also cover highlights of Process Analytical Technology (PAT) and Continuous Manufacturing (CM), which support the inclusion of QbD and vice versa.
Download the full article as PDF here Is it advantageous to use quality by design (QbD) to develop nanoparticle-based dosage forms for parenteral drug administration
or read it here
C. Camacho Vieira, L. Peltonen, A.P. Karttunen, A.J. Ribeiro, Is it advantageous to use quality by design (QbD) to develop nanoparticle-based dosage forms for parenteral drug administration?, International Journal of Pharmaceutics,
2024, 124163, ISSN 0378-5173, https://doi.org/10.1016/j.ijpharm.2024.124163.
Read also our introduction article on Parenteral Excipients here:


