Quality-by-Design-Based Development of Rivaroxaban-Loaded Liquisolid Compact Tablets with Improved Biopharmaceutical Attributes
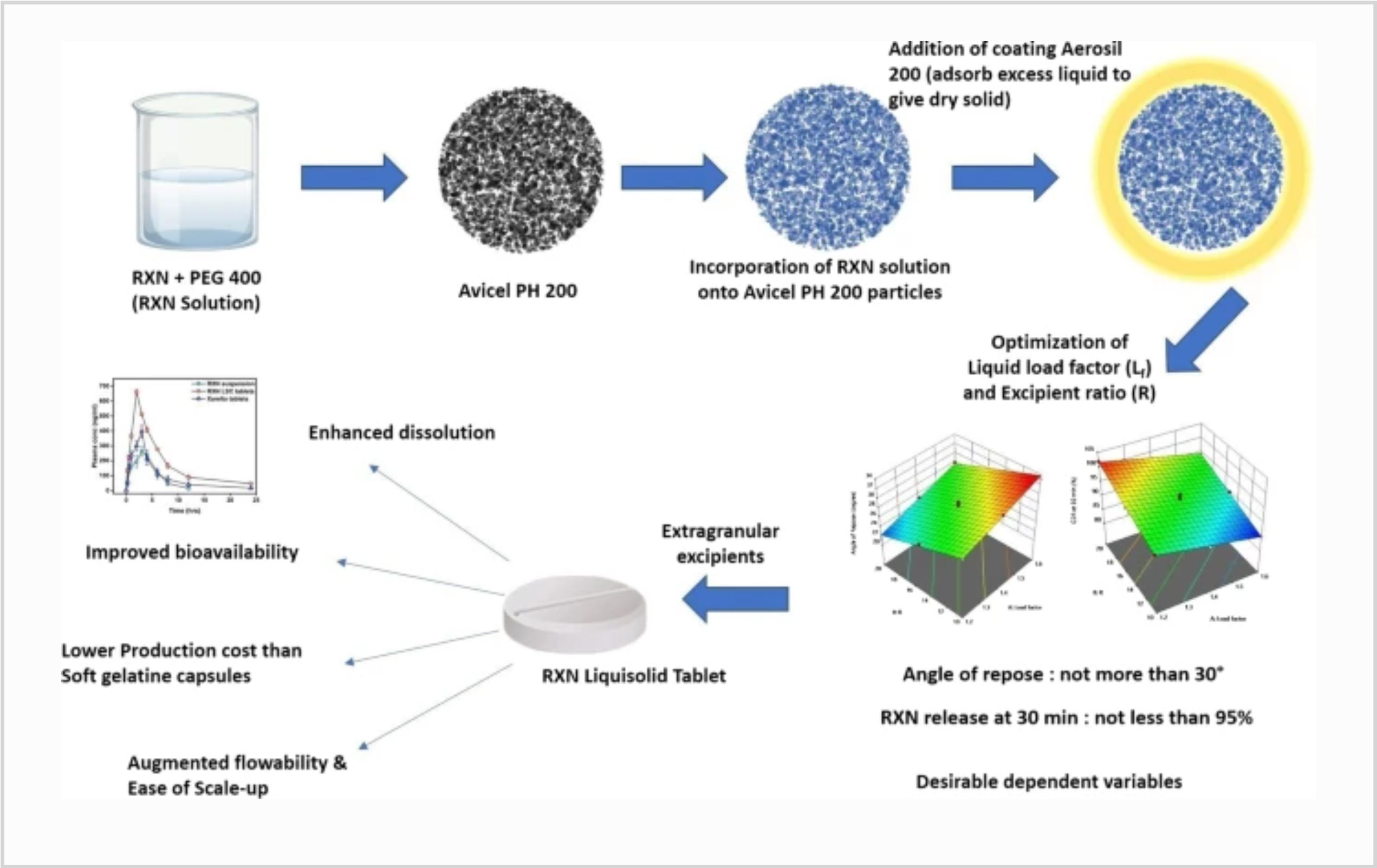
Rivaroxaban (RXN) finds use in the management of pulmonary embolism and deep vein thrombosis. Its poor solubility (5–7 µg/mL) and P-gp-mediated efflux from intestinal lining limits the oral application of RXN. This work assessed the impact of liquisolid compact technique in augmenting the solubility and bioavailability of RXN. PEG 400, Avicel PH 200, and Aerosil 200 were used as non-volatile liquid, carrier, and coating material, respectively, to formulate RXN liquid–solid compacts (RXN LSCs). A 32-factor factorial design was used in the optimisation to assess the impacts of factors (load factor and carrier:coating ratio) on the responses (angle of repose and Q30 min).
Pre-compression parameters of RXN LSCs suggested adequate flow and compressibility. Optimisation data suggested significant influence of factors on both the responses. Optimised RXN LSC-based tablets showed a significantly higher in vitro dissolution rate than RXN API and Xarelto® tablets due to improved solubility, reduced crystallinity, greater surface area, and enhanced wetting of RXN particles. XRD, DSC, and SEM data supported RXN’s amorphization. The cytotoxicity (MTT assay) and permeation studies indicated the nontoxicity of prepared RXN LSC tablets and the role of PEG 400 in inhibiting P-gp.
Pharmacokinetic study of RXN LSC-based tablets in Albino Wistar rats exhibited 2.51- and 1.66-times higher AUC in comparison to RXN API and Xarelto® tablets respectively, demonstrating that developed formulation had a greater oral bioavailability. The RXN LSC tablets showed longer bleeding times and higher rates of platelet aggregation than RXN API. Thus, RXN LSC tablets can be considered a facile, scalable technology.
Read more here
Excipients mentioned in the article beside others: Avicel PH 200, Aerosil 200, Soluplus, PEG 400
Shah, P., Desai, H., Vyas, B. et al. Quality-by-Design-Based Development of Rivaroxaban-Loaded Liquisolid Compact Tablets with Improved Biopharmaceutical Attributes. AAPS PharmSciTech 24, 176 (2023). https://doi.org/10.1208/s12249-023-02635-3
Read more on Quality by Design (QbD) here:


