Excipient Reactivity and Drug Stability in Formulations
This is about the excipient used for achieving sustained release function undergone chemical reaction with the stabilizing agent used for the drug product leading to product quality failure from the desired dissolution behavior. The generic version of Bupropion HCl SR 150 mg, i.e. sustained (12 hours) release tablet was made by matrix design using the excipient hydrophilic polymer Hydroxypropyl Cellulose; Klucel® HXF Pharm grade. The Exhibit Batch (Regulatory Lot) tablets of the generic Bupropion HCl SR 150mgRLD tablets at one month accelerated stability had a very high drug release within one hour (dose dumping) as opposed to pre-designed 12 hours sustained release property.
There was the pre knowledge that Bupropion HCl drug product needs a micro-environmental pH ≤ 5.0 for its stability. Thus, the matrix formulation may need the use of a stabilizer.
The following factors were the primary criteria for selecting a polymer:
- To select a patent non-infringing polymer [ i.e. no HPMC]
- To select a polymer that will be compatible with the low pH [£ 5.0] required for the Bupropion HCl stability.
- To select a polymer that has the similar type of matrix as brand for dissolution [brand has a swelling & erosion type matrix]. This will ultimately help in bio equivalency.
Technology
- Sustained-release hydrophilic matrix dosage form (tablet)by swelling and erosion (same as RLD)
Challenges Faced
- Finding a suitable polymer for the hydrophilic matrix dosage form
- Selecting an acidifying agent (buffer) that will keep product micro-environmental pH ≤ 5.0
- Use of dilute hydrochloric acid as a granulating fluid and its potential impact on corrosion of stainless steel equipment.
Formulation Approaches
Like RLD tablets generic SR tablets were made by matrix technology. The excipient hydrophilic polymer, a hydroxypropylcellulose Klucel® HXF Pharm grade was used as the polymer for the primary approach for a matrix tablet. A hydrophilic polymer, hydroxypropyl cellulose [HPC] with the property of swelling in water and forming a gelatinous like substance was considered for sustained drug release by both diffusion out of the gel and erosion of the tablet to have the in-vivo drug bio-equivalence.
During the development stage of this product, it was established that the this polymer is (a) compatible with the low pH (≤ 5.0) required for the Bupropion HCl stability and (b) the generic product exhibited a similar type of release behavior as that of the brand during dissolution testing.
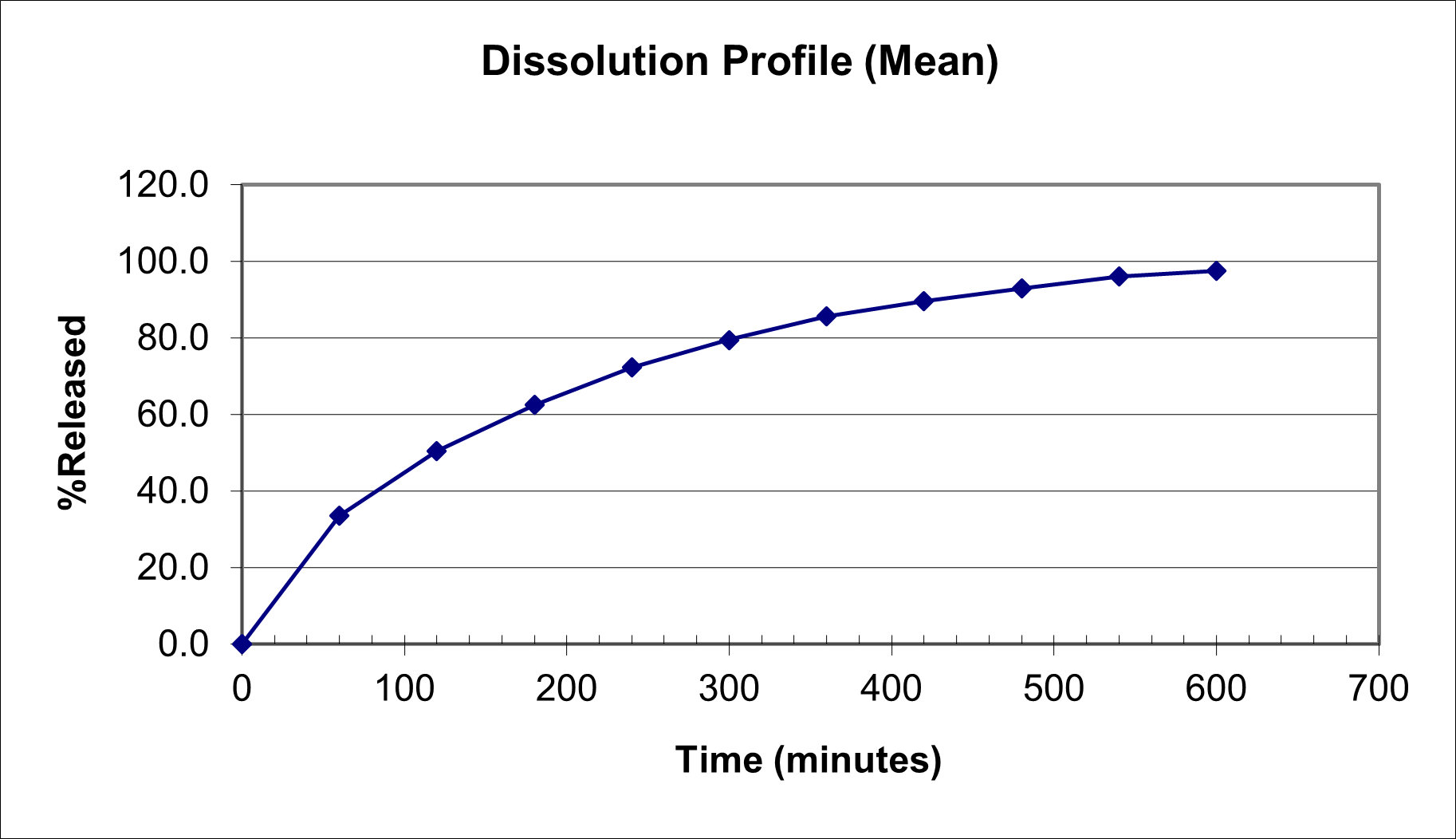
Three exhibit (Regulatory) batches (one of each, 100mg, 150mgRLD and 200mg) of Bupropion SR Tablets were manufactured. These initial tablets were analyzed for drug release from the dosage form. The dissolution profile was satisfactory with a desired profile where the drug release is controlled to release up to 10-12 hours.
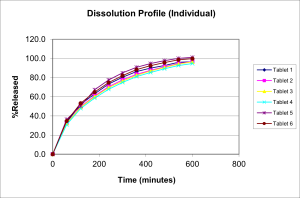
These tablets were in place for stability under the following conditions: Stress at 50C/75%, accelerated temperature of 40C/75% RH and Room Temperature at 25C/60% RH. Upon stability, the stress and accelerated samples were again tested for drug release. It was observed that in one of the stress sample (150mg at stress and accelerated condition sample) there was dose dumping during dissolution process, where almost all the active in the formulation was released within few minutes of the dissolution process instead of prolonged and slow release. However, the batch under room temperature behaved similar in terms of drug release as compared to the initial analysis.
So, a discrepancy was found in the stress samples of the 150mg batch with respect to the drug release during in-vitro dissolution process. Hence, an analysis of the result and problem was undertaken to understand and if possible solve the problem.
Thus, this dissolution failure at stability study needed further evaluation. Therefore, studies were required on the product formulation in conjunction with the use of HPC as the matrix polymer. Aqualon the manufacturer of the polymer hydroxypropyl cellulose was contacted. Aqualon and the Generic’s R&D conducted series of physico-chemical testing to investigate and find the root cause of dissolution failure and/or physical popping of the tablets in the dissolution apparatus.
Acid catalyzed hydrolysis is a mechanism that can occur with all cellulose ethers. Celluloses are sufficiently stable towards hydrolysis for most uses including film coating and wet granulation followed by oven drying at moderate temperature conditions e.g. 50-70 deg C. However, they can be susceptible to hydrolysis by acids and, to a lesser extent, by alkalis. Acids attack the acetyl linkages, cleaving the 1-4-glycosidic bonds. The hydrolysis mechanism is catalyzed by the presence of water and the rate is increased by increasing temperature. The result of hydrolysis is chain cleavage, which translates to a polymer with reduced molecular weight and reduced viscosity. Thus, there is a very fast in-vitro drug release.
It was found that the HCl used for wet granulation of the Bupropion HCl to stop its cyclisation has also degraded the polymer HPC HXF Pharm by hydroxylation reaction within the product during its storage for stability, resulted in breaking of the polymer Klucel® HXF Pharm grade. Thus resulted in dose dumping as opposed to providing sustained released. We consulted with the supplier of the excipient HPC Hercules Inc. (Aqualon) Dr. Tom Durig helped us for the investigation of viscosity and Gel Chromatography of Bupropion HCl SR formulation using other grades of HPC. Finally it was found using the lower molecular weight grade HPC EXF Pharm with more amount than the high molecular weight HPC-HXF Pharm grade. HPC EXF Pharm gave us the right formula with no dose dumping at the accelerated stability condition.
Original failed formula due to dose dumping at accelerated stability time points

Dissolution of 150mg clinical bio samples Vs RLD-bio tablets @ 100rpm
The dissolution results @ 100rpm of 150mg clinical samples are matching with the bio-reference tablets at same condition. Therefore, 150mg E. Batch tablet are equivalent in their in-vitro drug release with reference tablets.
Manufacturing Process and Probable Cause
During the manufacture process, the polymer is mixed with other formulation components in the presence of water and hydrochloric acid. During drying, the polymer was subsequently subjected to heat and humidity. It is possible to create drying conditions that may alter the morphological state of the polymer by sequestering molecules of water at the center of the polymer particles. This can significantly slow down the dissolution rate of the polymer. Should such conditions occur, the reduced dissolution rate of the polymer might affect the gel formation rate and the gel strength? The gel strength is very important in controlling the drug release from the matrix.
The above process may also affect the effective formation of integrated matrix, which is required for polymer function as a rate controlling excipient.
In addition, an analysis of formulation was conducted by GPC (Gel Permeation Chromatography) method. This testing process evaluates the molecular integrity (molecular weight) and quantity of the recoverable polymer in the formulation. One reason for the test was to determine if the HPC in the tablets might have undergone chain scission resulting in lower viscosity due to presence of unfavorable environmental conditions (e.g. heat, humidity, and pH). The test was performed on the following samples: Fresh HPC sample as a control, bad formulation under room temperature condition and bad formulation samples under stress condition. The test results indicated the following: The HPC polymer was intact and of similar molecular weight in both the fresh polymer sample and bad formulation under room temperature. However, on the bad formulation sample under stress condition, a large portion of high molecular weight polymer was missing. This percentage of polymer loss was between 25-40%. However, the “missing” fraction was not detected as shorter chain length HPC. Neither could it be accounted for in any other way. There is no simple explanation for this phenomenon. One possible explanation is that some polymer could have become insoluble, thus not extractable for analysis. A loss of “soluble” polymer content and integrity may have led to the dose-dumping phenomenon during the dissolution testing.
Finally, a potentiating effect of the following factors may have led to the failure of the HPC polymer to function as a rate controlling excipient:
- Over drying of the batch during the drying process.
- Drying in the presence of high percentage of humidity in the inlet air. The presence of a strong acid in the formulation.
- Finally, a relatively low percentage of polymer content in the formulation. These four factors may have combined to affect the formulation in a deleterious way
It was also indicated by the vendor, based on basic principles of reaction chemistry, that the samples (of the bad batch) in the room temperature condition would be expected to be less susceptible to dose dumping process as compared to the stress and accelerated samples. It was however, not possible to predict the kinetics and time point at which the room temperature sample may display a similar dose dumping process like the stressed sample. Thus, this type of stability test strategy carries an unknown level of risk.
Relationship between the high pH and dose dumping of accelerated sample is not conclusive as per the generated resulting data.
There are a number of variables, which would influence cellulosic hydrolysis:
1) Water – Water is needed to catalyze the reaction without the presence of water the hydrolysis reaction cannot precede. Increased moisture and humidity levels will increase the rate at which hydrolysis proceeds.
2) Temperature – It is possible that in the presence of dilute acid that at low temperatures (such as room temperature) one will not observe the hydrolysis mechanism either because it is not thermodynamically favorable or it is kinetics very slow. As one goes to higher temperature the rate of hydrolysis increases. This would mean that it is possible not to see a loss of polymer chain length at low temperature but only at higher temperatures. Temperature is secondary as a direct variable. The degradation kinetics are temp. dependent, but since everything is done at a defined relative humidity, the focus should be on the temperature dependence of the water load in the system, which just provides a greater reservoir of moisture to the system.
3) Time – If the hydrolysis reaction is thermodynamically possible then the time at which the polymer is subjected to the increased temperature conditions is a variable. The longer the polymer is exposed to the increased temperature conditions is the presence of the dilute acid and water at a high temperature the faster the hydrolysis rate.
In our opinion, the acidic environment with high temperature and humidity has triggered the break-down of HPC. This has caused decreased gel strength in the tablets, resulting into “dose dumping”. The Process Characterization (PC) lots did not exhibit this phenomenon even in three months accelerated stability samples. Looking into old stability samples this “dose dumping” has also been observed occasionally in the some of the development lots, in fact, one month sample (accelerated conditions) of lead formula batches did not exhibit this behavior. No further testing was performed on the development lots as the PC batches were put on stability studies.
Final Improved Formula with change of grade of HPC for stability
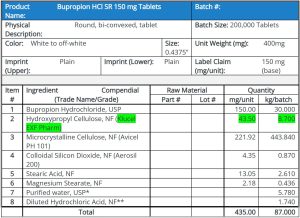
Process Technique for the new formulation
Previous formulation in which there was a dose dumping phenomenon had HPC of 5% intra-granular and 5% extra granular. Therefore, this 5% intra-granular HPC is more susceptible to acid catalyzed hydrolysis. In the new experimental design, a minimum quantity of the HPC in the intra-granular portion of the granulation is used. The reason is that more HPC extra granular will not be in contact with the dilute HCl solution and will also not be subjected to the drying process. This should improve the likelihood of the polymer not cleaving due to non-exposure to moisture; dilute acid, increased temperature or drying time. All of these as described earlier increase the rate of hydrolysis of the polymer.
Batches were made with HPC-EXF 1% intra granular and 9% extra granular in conjunction with reduced amount of diluted HCl to 2.0% w/w in the formula. Tablets made with this process were subjected to both stress (50°C/75%RH) and accelerated (40°C/75%RH) stability conditions. They were physico-chemically tested for both pH and dissolution behavior characteristics. Studies revealed that tablets of stress stability up to 2 weeks were holding together without any popping. Tablets of accelerated stability up to three months were not only holding together but also their dissolution profile matched with that of the initial time point.
Final confirmatory formula repeated on three process characterization batches to establish product/process parameters.
A ready-mix coating formula with the polymer HPC-LF (Opadry) used for the esthetic non-functional aqueous coating of the core tablets of all strength.
Read more here
Following excipients are mentioned in the study besides other: Klucel® HXF Pharm, Aqualon, Klucel EXF Pharm, Avicel PH 101, Aerosil 200, Magnesium Stearate
Source: APPS NEWSMAGAZIN, Dr. Masih Jaigirdar, Cover Article, April 2024, Excipient Reactivity and Drug Stability in Formulations – AAPS Newsmagazine
See our next webinar:
“Rethinking the development of controlled release formulations and manufacturing processes”
Date: 30th of April, Time: 3:00 pm (Amsterdam, Berlin)


