Outlining Successful Steps for Scale-Up and BLA Filings
Successful scale-up and BLA filings for products manufactured by microbial biomanufacturing require strategic planning.
In the high-stakes world of microbial biomanufacturing, there are dozens of critical moments and crucial decisions to be made on the path from clinical trials to regulatory approval to commercial manufacturing. Planning and executing both scale-up and a successful biologic licensing application (BLA), however, may be two of the most significant moments in the regulatory pathway.
First and foremost, the financial, intellectual, and time investment in developing a biopharmaceutical product through microbial fermentation is high. Tens and even hundreds of millions of dollars are at stake. Errors, lack of knowledge, and poor planning can have catastrophic effects on a project, leading to tens of millions of dollars lost or even projects being discontinued for budgetary reasons.
But a well-planned scale-up and soundly executed BLA, implemented with an experienced contract development and manufacturing organization (CDMO), can save pharmaceutical companies months of wasted time and money. When development timelines are measured in years and investments are measured in eight and 10 figures, planning and execution are of the highest importance.
Planning for scale-up and BLA
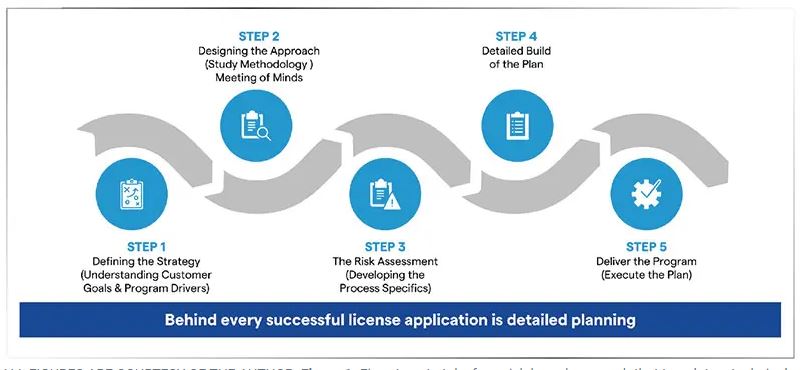
When moving from early stage proof-of-concept to clinical and then commercial stages, the crucial steps of scaling up a microbial product and garnering regulatory approval both become significant steps. Both these undertakings require a full understanding of the goals, the process, the facilities, the safety, and the demand for the bio/pharmaceutical by the manufacturer or CDMO and its partners. Scale-up planning and the development of the BLA work in concert to help all of the collaborators define, plan, forecast and execute the development of the product and reach its market potential (see Figure 1).
Once the proof-of-concept has been achieved, a manufacturer must develop a full understanding and plan for scale-up. All partners, which could include biotechs, bio/pharma companies, and CDMOs, must understand and agree on the goal, the process, the development plan (including potential deviations), production, and manufacturing. In addition, all partners should also be addressing questions about demand, scale, budget, and details of the supply chain.
Preparing to scale up and file for a BLA requires that the manufacturer answer these questions so they can test and understand the boundaries of their project’s parameters: process design, plant fit, process robustness parameter characterizations, and design space, to name a few. If the project isn’t properly planned, one might not be able to ramp up supply quickly enough due to a lack of capacity and/or throughput. If the manufacturer scales too high, then it will build up inventory, and the shelf-life or stability of the product may be at risk. That product may need to be scrapped, which is an expensive error. Conversely, if the scale is too low, the manufacturer might not be able to produce enough product or substance, which results in a loss of market opportunity.
S. Varray, “Outlining Successful Steps for Scale-Up and BLA Filings,” BioPharm International 35 (8) 22–26 (2022).

