Flexible Coatings Facilitate pH-Targeted Drug Release via Self-Unfolding Foils: Applications for Oral Drug Delivery
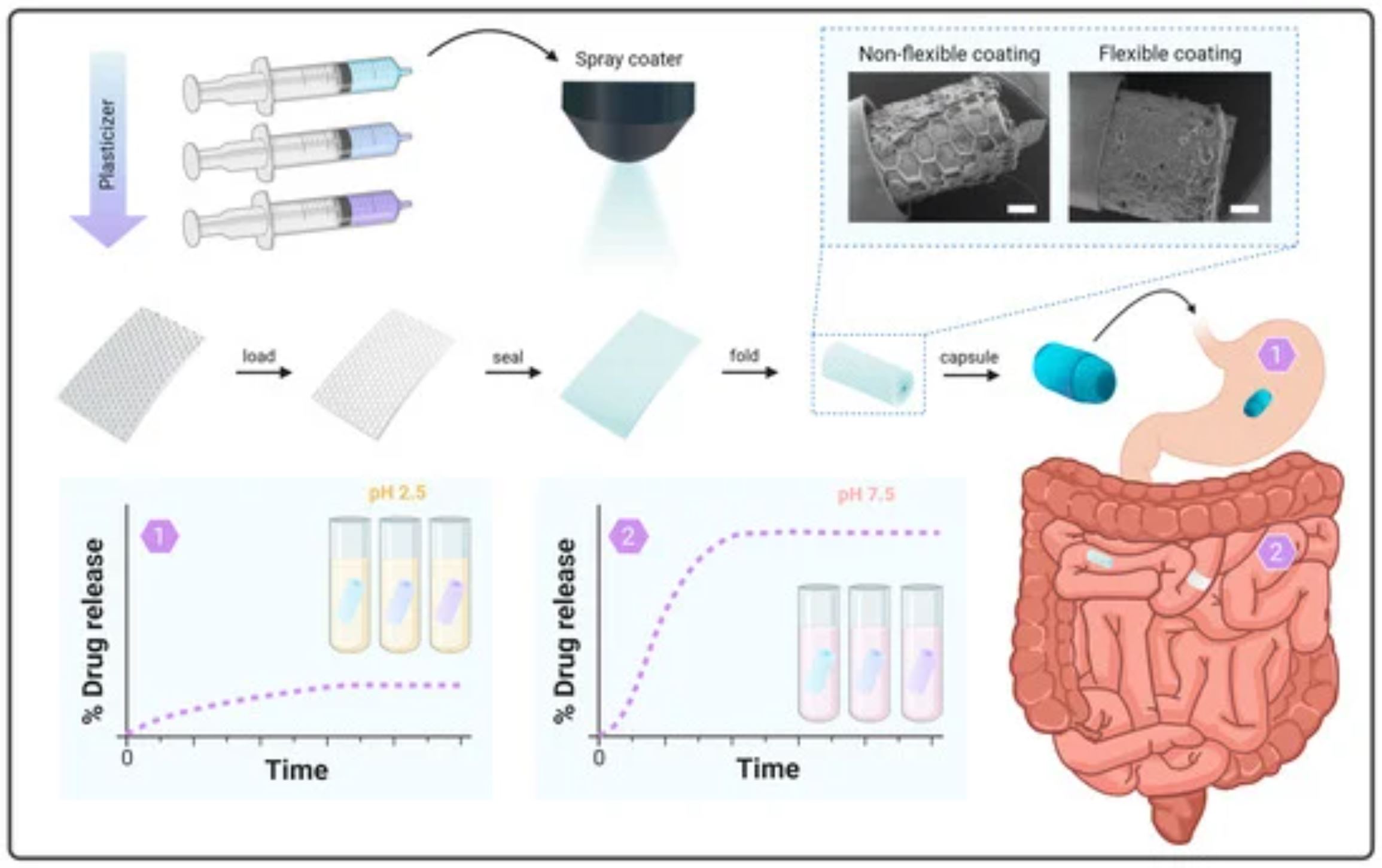
Ingestible self-configurable proximity-enabling devices have been developed as a non-invasive platform to improve the bioavailability of drug compounds via swellable or self-unfolding devices. Self-unfolding foils support unidirectional drug release in close proximity to the intestinal epithelium, the main drug absorption site following oral administration. The foils are loaded with a solid-state formulation containing the active pharmaceutical ingredient and then coated and rolled into enteric capsules. The coated lid must remain intact to ensure drug protection in the rolled state until targeted release in the small intestine after capsule disintegration. Despite promising results in previous studies, the deposition of an enteric top coating that remains intact after rolling is still challenging.
In this study, we compare different mixtures of enteric polymers and a plasticizer, PEG 6000, as potential coating materials. We evaluate mechanical properties as well as drug protection and targeted release in gastric and intestinal media, respectively. Commercially available Eudragit® FL30D-55 appears to be the most suitable material due to its high strain at failure and integrity after capsule fitting. In vitro studies of coated foils in gastric and intestinal media confirm successful pH-triggered drug release. This indicates the potential advantage of the selected material in the development of self-unfolding foils for oral drug delivery.
Download the full article as PDF here Flexible Coatings Facilitate pH-Targeted Drug Release via Self-Unfolding Foils
or read it here
Materials
A SylgardTM 184 silicone elastomer kit was bought from Dow Chemical (Midland, MI, USA). Eudragit® L100-55 (EL100-55) and Eudragit® FL30D-55 (EFL) were both obtained from Evonik (Essen, Germany), whereas Kollicoat® MAE 100-55 (KMAE100-55) was acquired from BASF (Ludwigshafen, Germany). Polyethylenglycol (PEG) 6000 (MW 7000–9000 g/mol), which presents a polydispersity of 1 and Mw 6019 g/mol according to the literature, was acquired from Merck (Darmstadt, Germany) [32]. Paracetamol (98–102% purity) and poly(vinyl alcohol) (98–99% hydrolyzed), as well as PBS tablets, were purchased from Sigma-Aldrich (St. Louis, MO, USA). Isopropanol (IPA) and acetone were purchased from VWR International (Radnor, PA, USA). Ultrapure water used throughout the studies was obtained from a Q-POD dispenser from Merk Millipore (Burlington, MA, USA).
Milián-Guimerá, C.; De Vittorio, L.; McCabe, R.; Göncü, N.; Krishnan, S.; Thamdrup, L.H.E.; Boisen, A.; Ghavami, M. Flexible Coatings Facilitate pH-Targeted Drug Release via Self-Unfolding Foils: Applications for Oral Drug Delivery. Pharmaceutics 2024, 16, 81. https://doi.org/10.3390/pharmaceutics16010081
Read more on “Orally Disintegrating Tablets (ODTs)” here:


