Silicified Microcrystalline Cellulose, Modern Co-Processed Excipient For Low Dose Solid Dosage Forms
ABSTRACT
Low dose 75 mg aspirin tablets are widely used for adults in arthritis, rheumatic fever, pain and to reduce uric acid
excretion. Low dose formulation are manufactured by wet granulation method, which is time consuming and
laborious. However these days direct compression method is in trend. Direct compression method is cost effective
and gives better productivity than wet granulation method. Microcrystalline cellulose and silicified microcrystalline cellulose are suitable excipients for direct compression method. Silicified microcrystalline cellulose is
favorable for low dosage forms. It controls drug content uniformity in the tablets. Silicified microcrystalline
cellulose has outstanding flow-ability, when it is mixed with API, material fills into die equally during tableting.
In this study, we compared SEM analysis, flow-ability and tablet profile of Silicified microcrystalline cellulose
with microcrystalline cellulose. We used HiCelTM MCC and HiCelTM SMCC to evaluate this study. Physical
properties of HiCel™SMCC are novel for low dose tablet.
INTRODUCTION
Aspirin comes under NSAIDs (Nonsteroidal anti-inflammatory drugs and antipyretic analgesics) drugs, it is acetyl derivative and chemical name is Acetylsalicylic acid. [1] It is used as analgesic, as anti-inflammatory, antipyretic and inacute rheumatic fever, rheumatoid arthritis and osteoarthritis. Aspirin is used as OTC (over the counter) drugs. [2] Two different low dosage aspirin tablets 75 mg and 81 mg are available in the market. [3] Low dosage Aspirin tablets can be manufactured by either wet granulation or roller compaction or direct compression (DC). Wet granulation method is very popular and old method for low dosages form. Direct compression method is used in pharmaceutical industries for making low dose, medium dose and high dose because direct compression is convenient and fast. [4] Without best quality excipients support, DC could be a difficult method for manufacturing tablets. It may deviate tablet profile parameters viz. Hardness, Friability and Disintegration time. [4] However, many pharmaceuticals excipients i.e. microcrystalline cellulose, silicified microcrystalline cellulose and lactose directly support direct compression method. These excipients improve the tablet profile such as weight uniformity, hardness, friability, disintegration time and dissolution profile.[5]
Microcrystalline cellulose belongs to cellulose group. It is a high degree polymer and through controlled hydrolysis, it converts to low degree polymer. Hydrolysis reaction is done in presence of mineral acid and neutralized with ammonia solution. Spray dried material in white color, granular, free flowing powder. [6] Different grades of MCC are available in market, which are used to manufacturing both DC and wet granulation formulation. [7] However, with some API’s, there are functional problems like poor flow, reduced surface area for bonding etc, which could result in poor quality of tablets. To eliminate the functional problems outlined, it is suggested to use co-processing of MCC with other established excipients. [5] Co-processing MCC may improve the preference of materials which is used in direct compression. There are many co-processing exipients used to manufacture the direct compression formulation. This process produces a material with beneficial characteristics with respect to flowability, mechanical strength and disintegration. [8]
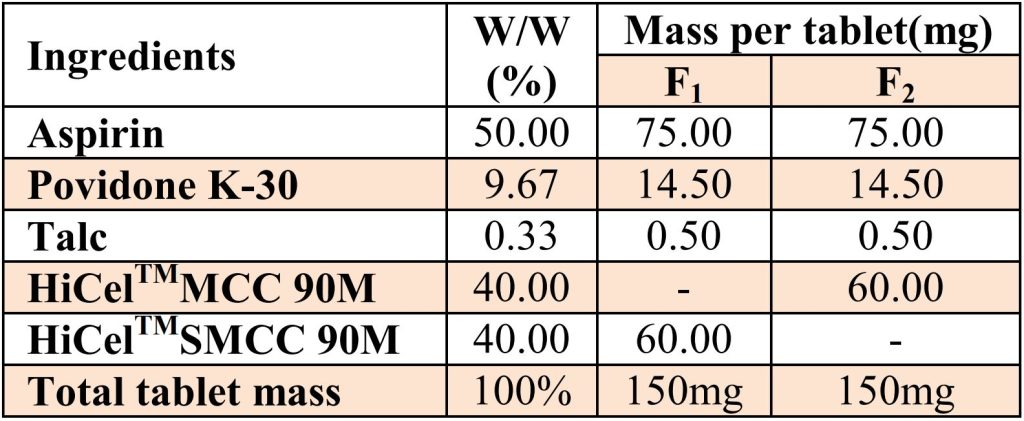
Table No-1: Dispersible Aspirin tablet manufacturing formula.
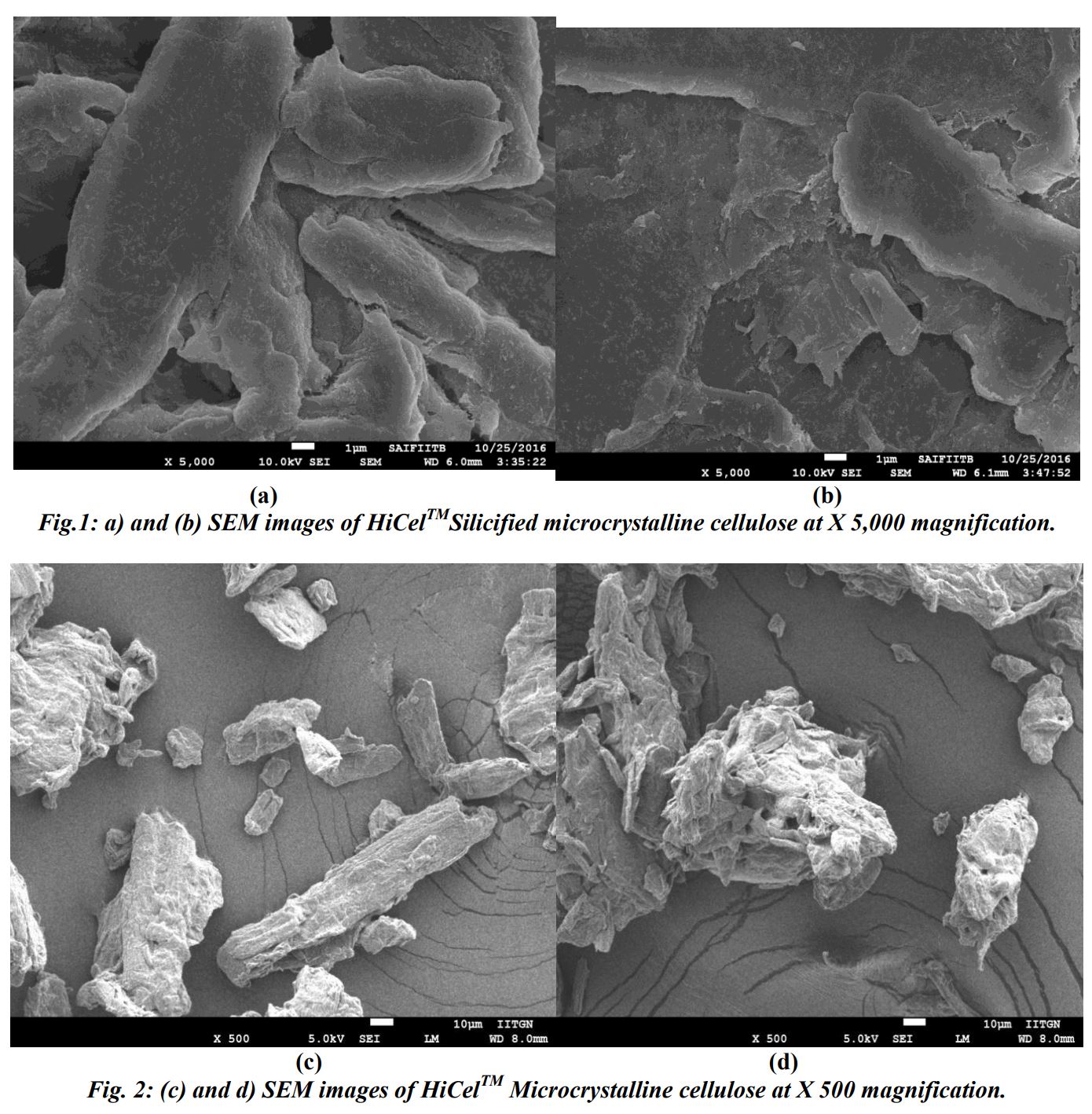
Silicified microcrystalline cellulose excipient is co-processed MCC. It is a combination of 98 % microcrystalline cellulose and 2% colloidal silicon dioxide, through wet mixing and dried by spray dryer. [9] Silicified microcrystalline cellulose has excellent flow properties and gives satisfactory tensile strength on less compaction force. In low dosages solid formulation it gives excellent weight uniformity. It absorbs very less moisture from environment. [10]
The main aim of study is find to direct compressible excipient for low dosages formulations. In this study comparative analysis of SEM, physical profile and tableting profile are done. Flow ability of both excipients HiCelTM SMCC and HiCelTM MCC are tested by FT4 powder rheometer. Flowability is represented by flow fraction (ffc), shear stress, Mohr’s stress circle and angle of repose. Select. Low dose Aspirin tablets are manufactured using two different excipients i.e. HiCelTM MCC and HiCelTM SMCC by direct compression.
Download the PDF here Silicified Microcrystalline Cellulose, Modern Co-Processed Excipient For Low Dose Solid Dosage Forms
Source: Sigachi, Silicified Microcrystalline Cellulose, Modern Co-Processed Excipient For Low Dose Solid Dosage Forms, Monika Tomar
Do you need more information or a sample of Sigachi excipients?

