A Quality by Design Approach for Developing SNEDDS Loaded with Vemurafenib for Enhanced Oral Bioavailability
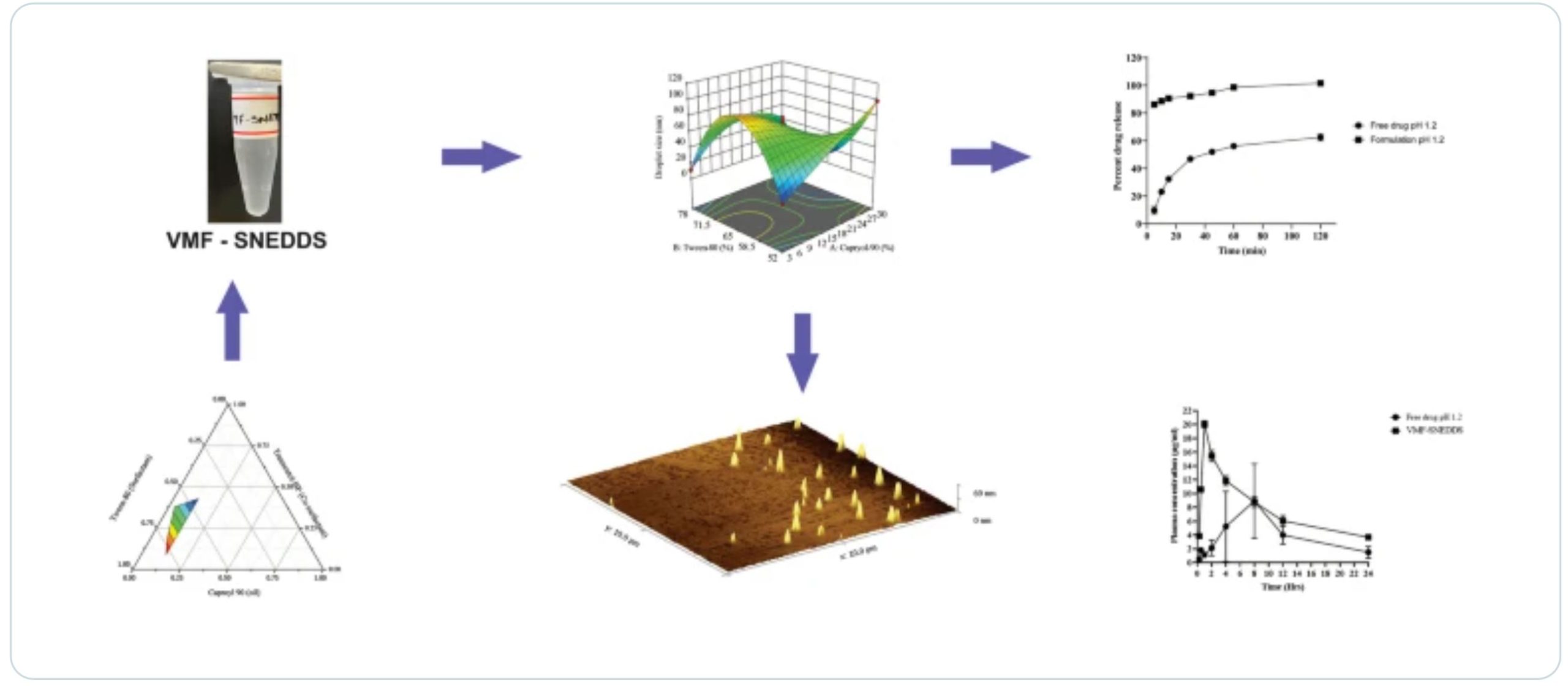
Vemurafenib (VMF) is a practically insoluble (< 0.1 μg/mL) and least bioavailable (1%) drug. To enhance its oral bioavailability and solubility, we formulated a reliable self-nano emulsifying drug delivery system (SNEDDS). A Quality by Design (QbD) approach was used to optimize the ratio of Capryol 90, Tween 80, and Transcutol HP. VMF-loaded SNEDDS was characterized for its size, polydispersity index (PDI), zeta potential, drug content, and transmittance. The in vitro release profile of the drug loaded in SNEDDS was compared to the free drug in two media, pH 6.8 and 1.2, and the data obtained were analyzed with different mathematical models.
A reverse-phase ultra-pressure liquid chromatography (UPLC) technique with high sensitivity and selectivity was developed and validated for the quantification of VMF in analytical and bioanalytical samples. Dissolution efficiency for SNEDDS was estimated using different models, which proved that the developed novel SNEDDS formulation had a better in vitro dissolution profile than the free drug. A 2.13-fold enhanced oral bioavailability of VMF-loaded SNEDDS compared to the free drug demonstrates the superiority of the developed formulation. This work thus presents an overview of VMF-loaded SNEDDS as a promising alternative to improve the oral bioavailability of the drug.
Read more here
JVUS, C., Kothuri, N., Singh, S. et al. A Quality by Design Approach for Developing SNEDDS Loaded with Vemurafenib for Enhanced Oral Bioavailability. AAPS PharmSciTech 25, 14 (2024). https://doi.org/10.1208/s12249-023-02725-2
Watch our free webinar series of Quality By Design (QbD) here:


