Understanding the Impact of Formulation Design on Microstructure and Drug Release from Porous Microparticle-Based Tretinoin Topical Gels
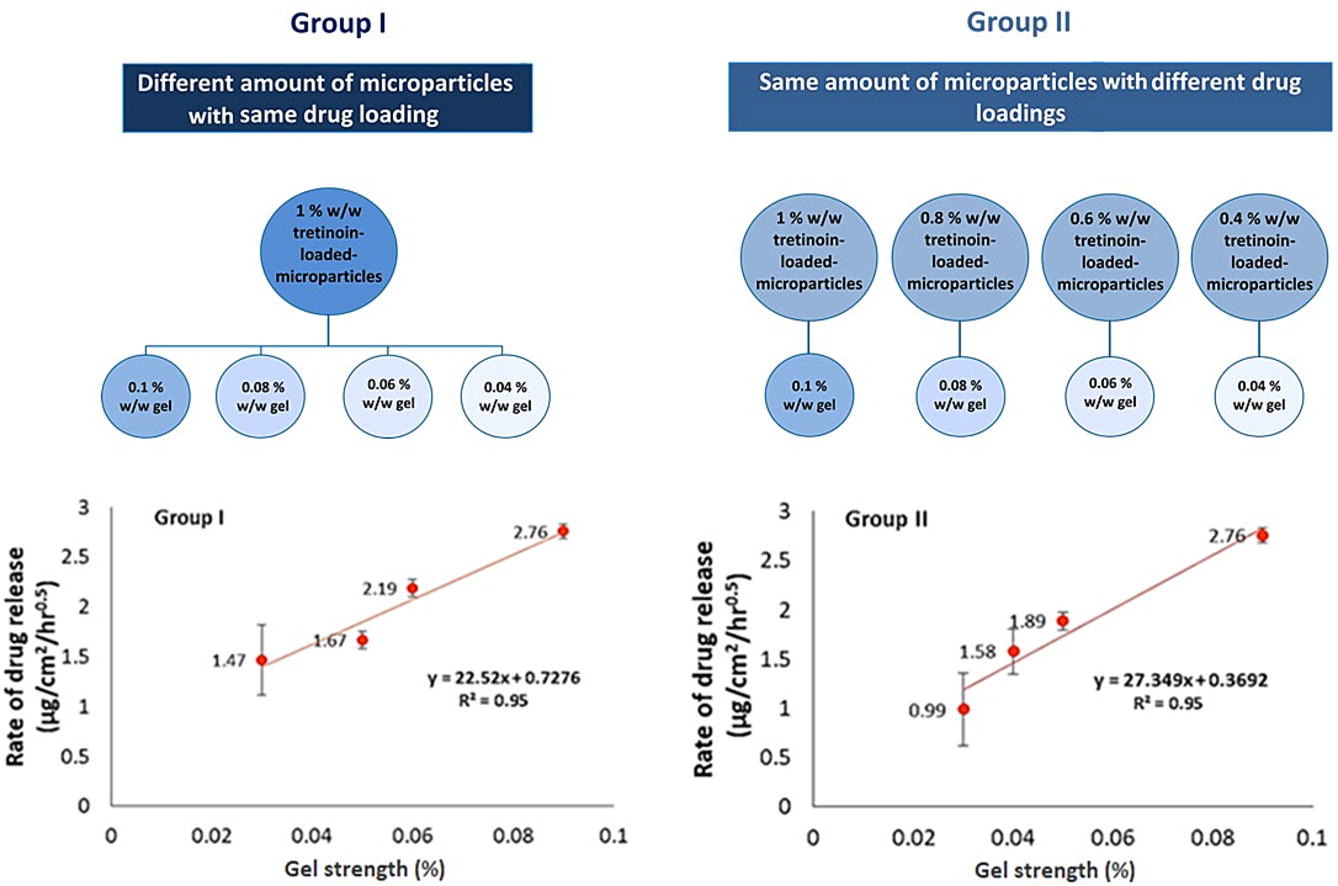
For proportionally formulated intermediate strengths of a topical product, the relationship of drug release across multiple strengths of a given product is not always well understood. The current study aims to assess the proportionality of tretinoin release rates across multiple strengths of tretinoin topical gels when manufactured using two different methods to understand the impact of formulation design on drug product microstructure and tretinoin release rate. Two groups of tretinoin gels of 0.04%, 0.06%, 0.08% and 0.1% strengths were manufactured. Gels in Group I were prepared by incorporating 4-10% g/g of 1% w/w tretinoin-loaded microparticles into a gel base. Gels in Group II were manufactured using 10% g/g of the microparticles that were loaded with increasing amounts (0.4-1% w/w) of tretinoin.
The two groups of gels were characterized by evaluating microstructure using a polarized microscope, rheology using an oscillatory rheometer, and drug release using Vison® Microette™ Hanson vertical diffusion cells. The microscopic images were used to discriminate between the two groups of gels based on the abundance of microparticles in the gel matrix observed in the images. This abundance increased across gels of Group I and was similar across gels of Group II. The rheology parameters, namely viscosity at a shear rate of 10 s-1, shear thinning rate, storage, and loss modulus, increased across gels of Group I, and were not significantly different across gels of Group II.
The release rate of tretinoin from the drug products was proportional to the nominal strength of the drug product in both Group I and Group II, with a correlation coefficient of 0.95 in each case, although the absolute release rates differed. Overall, changing the formulation design of tretinoin topical gels containing porous microparticles may change the physicochemical and structural properties, as well as the drug release rate of the product. Further, keeping the formulation design consistent across all strengths of microparticle-based topical gels is important to achieve proportional release rates across multiple strengths of a given drug product.
Read the full article here
Materials
Blank microparticles 5640 were purchased from AMCOL Health & Beauty Solutions (Lafayette, LA). Tretinoin reference standard powder (USP), sodium azide, BRIJTM O20 (Oleth-20), butylated hydroxytoluene (BHT), ascorbic acid, acetone, acetonitrile (HPLC grade), Nuclepore Track-Etched synthetic membranes, acetic acid, benzyl alcohol, carbomer homopolymer type B (Carbopol 974P), edetate disodium, glycerin, propylene glycol, stearic acid, sorbic acid, trolamine were purchased from Millipore Sigma.
Khaled H. Elfakhri, Mengmeng Niu, Priyanka Ghosh, Tannaz Ramezanli, Sam G. Raney, Nahid Kamal, Muhammad Ashraf, Ahmed S Zidan, Understanding the Impact of Formulation Design on Microstructure and Drug Release from Porous Microparticle-Based Tretinoin Topical Gels, International Journal of Pharmaceutics, 2024, 123794, ISSN 0378-5173, https://doi.org/10.1016/j.ijpharm.2024.123794.
Read more on Stearic Acid as a pharmaceutical Excipient here:


