Virodex™ TXR-1 and TXR-2: Safe and effective viral inactivation and cell lysis

The REACH* addition of Triton™ X-100 to the candidate list of Substances of Very High Concern (SVHC), and its subsequent ban in Europe, left biopharmaceutical manufacturers seeking viable alternatives to this essential bioprocessing chemical. Explore the Virodex™ range for viral inactivation and cell lysis: REACH-compliant, sustainable materials that have been optimised for use in the biomanufacturing of recombinant proteins, blood fractionation products, viral vector-based therapies and more.
Features and benefits:
- Sustainable, REACH-compliant, and cGMP EXCiPACT manufactured
- Compendial grade, with known parenteral applications**
- Known use of chemistries by industry for viral inactivation1
- Equivalent performance to Triton™ X-100, with better performance than three other replacements on the market
- No risk of nitrosamine formation
- Improved biologic quality and lower biomanufacturing risk, enabling faster speed to market
- Offered in a range of pack sizes, including single-use
- Comes with additional COA testing: bacterial endotoxin, Total Aerobic Microbial Count (TAMC), and Total Yeast and Mould Count (TYMC) to support use in sterile manufacture
- Analytical methods available to confirm detergent removal in finished biopharmaceutical products
Why consider alternatives to Triton™ X-100?
Triton™ X-100 is a non-denaturing, non-ionic detergent that has been widely used for decades in various industries, including biopharmaceutical manufacturing. Triton™ X-100 is used to inactivate lipid-enveloped viruses, such as hepatitis viruses B and C and human immunodeficiency virus (HIV), in plasma derivatives and biopharmaceuticals produced by mammalian cell culture. Triton™ X-100 is also frequently employed as a cell lysis agent to release therapeutic molecules from cells. With evidence of their toxicity to aquatic life via endocrine disrupting activity, phenol ethoxylates were added to the to the European Chemicals Agency (ECHA) candidate list of Substances of Very High Concern (SVHC) under the REACH regulation in 2012. 2 Substances on this list are of very high concern due to their potential impacts on human health and the environment. The sunset date for the ban of Triton™ X-100 in Europe occurred on January 4th, 2021, prohibiting its use for most applications unless granted an exemption.3,4
Sustainable, compendial, cGMP EXCiPACTmanufactured, REACH-compliant chemicals
In response to concerns about potential future restrictions on its use, biopharmaceutical manufacturers are turning to alternatives to Triton™ X-100. When searching for replacements, biomanufacturers look for a product that is more sustainable, and is free of the same safety or environmental concerns associated with Triton™ X-100. This is the basis on which we have developed our Virodex™ range of detergents for viral inactivation and cell lysis: sustainable, compendial, cGMP EXCiPACT-manufactured, and REACH-compliant chemicals.
Croda Pharma’s Virodex™ range for viral inactivation and cell lysis
Under our Virodex™ range, we currently offer two distinct, well-studied chemistries: Virodex™ TXR-1 and Virodex™ TXR-2. Both products provide efficient viral inactivation and cell lysis by similar mechanisms, and with equivalent performance to Triton™ X-100. Virodex™ detergents are offered in single-use packs to meet the volume requirements for viral inactivation and cell lysis applications and come with additional COA testing for bacterial endotoxins, TAMC, and TYMC. Continue reading to learn how our sustainable, compendial, REACH-compliant, cGMP EXCiPACTmanufactured chemicals are used in the manufacturing of biologic drugs.
Identification of Triton™ X-100 replacements
Virus inactivation is a key step in the downstream processing of biopharmaceutical products to eliminate potentially harmful viruses or viral contaminants that could infect patients, ensuring the safety and efficacy of the final biopharmaceutical formulation. Detergent treatment aims to achieve a 10,000- to 100,000-fold reduction in viable virus particles (i.e., a log 4 to log 5 reduction). To identify nextgeneration detergents for biopharmaceutical manufacturing applications, 31 detergents belonging to 11 detergent classes were screened for equivalent virus inactivation activity to Triton™ X-100. This was achieved using xenotropic murine leukaemia virus (XmuLV), an established model lipid enveloped virus frequently used to assess detergents for virus inactivation. This research identified two detergents, Virodex™ TXR-1 and TRX-2, that exhibited equivalent performance characteristics to Triton™ X-100.
Virodex™ detergents for viral inactivation
To characterise the virus inactivation functionality of Virodex™ TXR-1 and TRX-2 the potency and kinetics of
virus inactivation were assessed using the XmuLV model of lipid-enveloped virus inactivation. In this assay, XmuLV
preparations were treated with detergent prior to infecting a sensitive cell line (Felis catus PG-4 ATCC CRL-2032). The
viral titer of detergent-treated and untreated XmuLV preparations was determined and the reduction in infectious
viral particles was calculated using the equation:
Potency determination: dose response assay
To assess the potency of the detergents for viral inactivation (i.e., the minimum concentration of detergent required to
achieve maximum virus inactivation) XmuLV was treated with detergents at 0.2%, 0.1%, 0.05%, 0.025% and 0.00625% for 2 h at 22°C prior to infecting F. catus PG-4 cells. As shown in Table 1, both Virodex™ TXR-1 and Virodex™ TXR-2 were as potent as Triton™ X-100, achieving maximum virus inactivation at 0.025%.
Virus inactivation kinetics
The time required to achieve a LRF of 4 to 5 is an important characteristic of a detergent used in downstream processing applications. To determine the kinetics of virus inactivation by Virodex™ TXR-1 and Virodex™ TXR-2, inactivation of XmuLV was assessed at three time points (1 min, 15 min, 120 min). Both Virodex™ detergents showed equivalent virus inactivation as Triton™ X-100 after a 15 min treatment time (Figure 1).
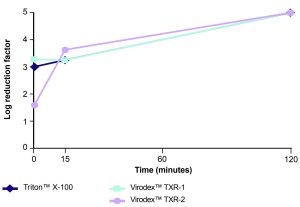 Figure 1. Virus inactivation kinetics. XmuLV was treated with each detergent at a concentration of 0.1% at 22°C and aliquots were removed at 1 min, 15 min and 120 min and used to infect F. catus PG-4 cells.
Figure 1. Virus inactivation kinetics. XmuLV was treated with each detergent at a concentration of 0.1% at 22°C and aliquots were removed at 1 min, 15 min and 120 min and used to infect F. catus PG-4 cells.Virodex™ detergents for cell lysis
In the production of biopharmaceuticals, Triton™ X-100 is used to lyse cells to extract biomolecules such as therapeutic proteins or viral vectors from production cell lines. For this reason, the effectiveness of Virodex™ detergents for cell lysis was evaluated in two industrially relevant cell lines, Chinese Hamster Ovary (CHO-K1) and Human Embryonic Kidney (HEK-293T) cells. Cultured cells were treated with each detergent over a wide range of concentrations (0.00015% – 1%) for 2 h at 22°C. To assess lysis, cells were stained with fluorescent stains that stain both the cell membrane and nucleus and cellular lysis was assessed by fluorescent microscopy. Lysis was evident by disintegration of the cell membrane (green) and nucleus (red) in acquired images (Figure 2 and Figure 3). The lowest detergent concentration required to achieve lysis of CHO-K1 cells was identical between the Virodex™ detergents and Triton™ X-100 (Table 2; Figure 2).
Source: Croda brochure “Virodex™ TXR-1 and TXR-2”
Do you need more information or a sample of Croda excipients?