Characterization of the viscoelasticity of pharmaceutical tablets using impulse excitation technique
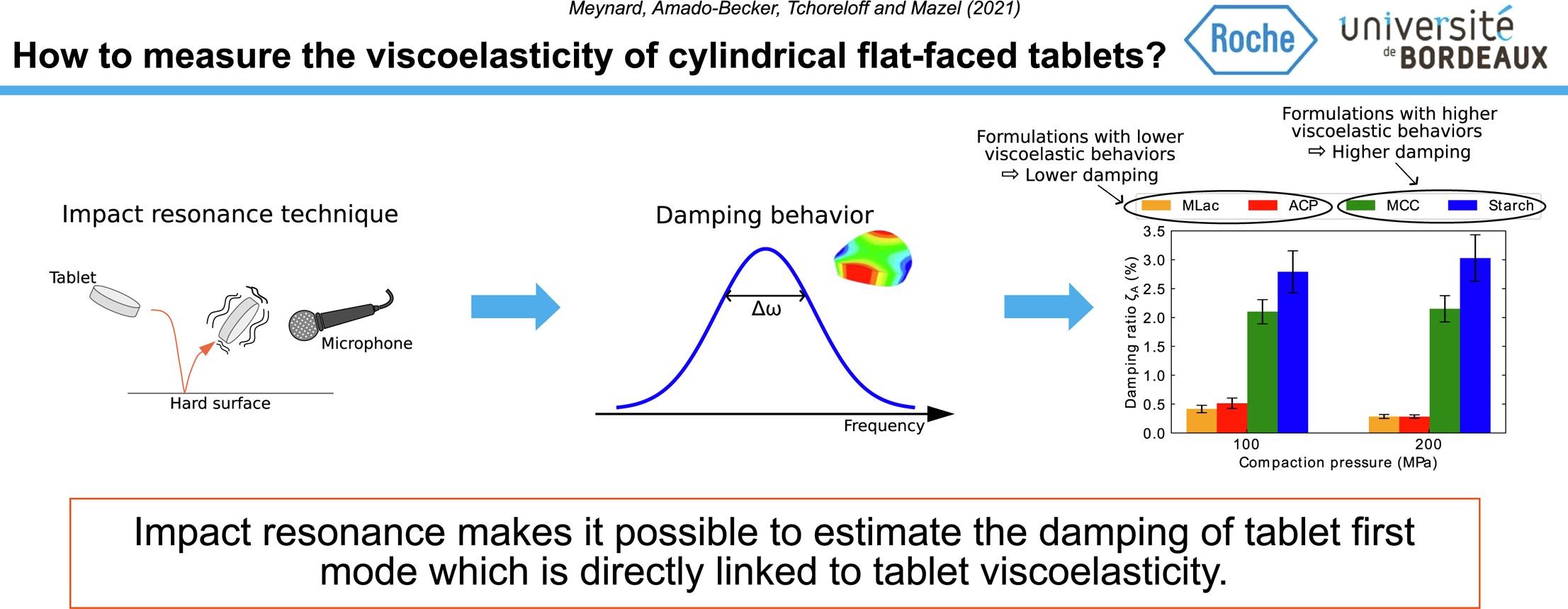
Pharmaceutical tablets can be produced on different kinds of presses that may have very different compaction kinematics. Strain rate sensitivity (SRS) is thus an important property for the powders used to produce pharmaceutical tablets. Viscoelasticity is one of the aspects of the SRS and can be sometimes difficult to characterize. In this work, impulse excitation technique was used as an easy-to-implement method for characterizing viscoelasticity using the fact that this property induces damping which can be detected on resonance spectra as peak enlargements.
A damping ratio, related to the first flexural vibration mode, was determined on impulse excitation frequency spectra using the half-power bandwidth method on tablets made with different products. This method made it possible to obtain reproducible results for the damping ratio. As viscoelasticity is not the only phenomena that can promote damping, tests were made in order to assess the influence of other parameters: viscoplasticity, porosity and tablet dimensions. Results indicated that the influence of these phenomena could be considered as negligible.
Finally, the damping ratios determined were in good accordance with the known viscoelastic behavior of the studied products. This made it possible to confirm that impact resonance is an easy and quick way to characterize the viscoelastic nature of pharmaceutical tablets.
About this article: J. Meynard, F. Amado-Becker, P. Tchoreloff, V. Mazel, Characterization of the viscoelasticity of pharmaceutical tablets using impulse excitation technique, International Journal of Pharmaceutics, Volume 613,
2022, 121410, ISSN 0378-5173, https://doi.org/10.1016/j.ijpharm.2021.121410. (https://www.sciencedirect.com/science/article/pii/S0378517321012163)
Materials
Four classical pharmaceutical excipients for direct compression were used in this study: lactose monohydrate “MLac” (Excipress GR150, ArmorPharma, Maen Roch, France), anhydrous dicalcium phosphate “ACP” (Di-cafos A60, Chemische Fabrik Budenheim, Budenheim, Germany), microcrystalline Cellulose “MCC” (Vivapur12, JRS Pharma, Weissenborn, Germany) and starch (Lycatab C, Roquette, Lestrem, France). Magnesium stearate (Partek Mg Lub, Merck, Darmstadt, Germany) was used for external lubrication during tablet manufacturing. In addition, the apparent particle density of each powder was determined using a helium pycnometer (AccuPyc 1330, Micromeritics, Norcross, GA, USA). The measurements were performed in triplicate (10 purges and 10 runs).


