A sterilizable platform based on crosslinked xanthan gum for controlled-release of polymeric micelles: Ocular application for the delivery of neuroprotective compounds to the posterior eye segment
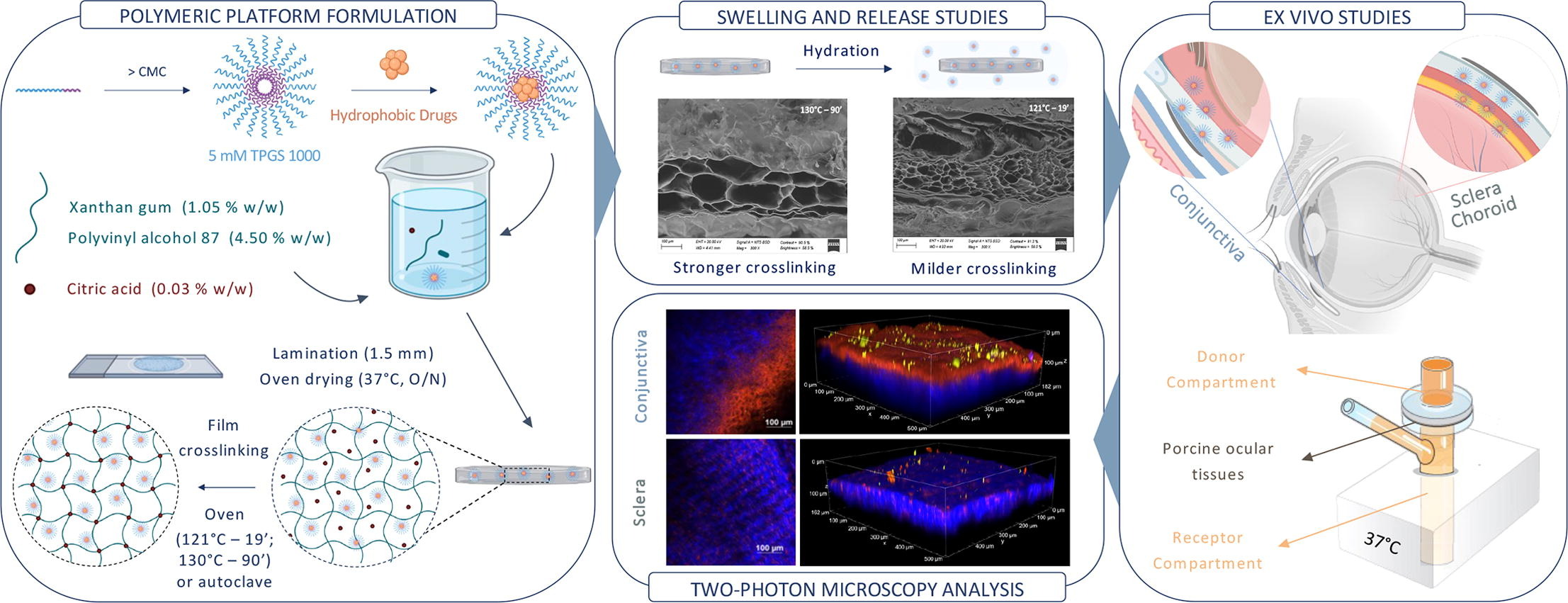
Abstract
TPGS (D-α-tocopheryl polyethylene glycol 1000 succinate) polymeric micelles show interesting properties for ocular administration thanks to their solubilization capability, nanometric size and tissue penetration ability. However, micelles formulations are generally characterized by low viscosity, poor adhesion and very short retention time at the administration site. Therefore, the idea behind this work is the preparation and characterization of a crosslinked film based on xanthan gum that contains TPGS micelles and is capable of controlling their release. The system was loaded with melatonin and cyclosporin A, neuroprotective compounds to be delivered to the posterior eye segment. Citric acid and heating at different times and temperatures were exploited as crosslinking approach, giving the possibility to tune swelling, micelles release and drug release. The biocompatibility of the platform was confirmed by HET-CAM assay. Ex vivo studies on isolated porcine ocular tissues, conducted using Franz cells and two-photon microscopy, demonstrated the potential of the xanthan gum-based platform and enlightened micelles penetration mechanism. Finally, the sterilization step was approached, and a process to simultaneously crosslink and sterilize the platform was developed.
Introduction
Retinal diseases such as age-related macular degeneration (AMD), diabetic retinopathy and glaucoma account for the majority of irreversible blindness cases worldwide (Health Organization, n.d.). The treatment of these diseases is very problematic, since, at the moment, there are limited – if any – therapeutic tools. While diabetic retinopathy and wet-AMD are controlled with corticosteroids and anti-vascular endothelial growth factor (anti-VEGF) compounds, dry-AMD cannot be treated. Glaucoma as well cannot be cured and it is only managed by lowering intraocular pressure. Despite presenting multiple and different pathogenic mechanisms, often not entirely clear, these pathologies have as common outcome the oxidative stress and the apoptosis of different retinal cells (Pardue and Allen, 2018; J. Wang et al., 2022). Several evidences suggest that neuroprotective compounds could contribute to preserve the patient’s current visual function promoting neuronal survival (Pardue and Allen, 2018), but their administration still represents a challenge. Indeed, the delivery of actives to the retina is very difficult due to numerous static and dynamic barriers that protect the inner structures of the eye (Nayak and Misra, 2018). For this reason, drugs – when available – are typically administered by intravitreal injections. This invasive route is associated with poor patient compliance and side effects, aggravated by the high administration frequency required for chronic pathologies (Thrimawithana et al., 2011). Therefore, an effective and less invasive administration strategy for targeting the posterior eye segment represents one of the most relevant medical needs in the ophthalmology field (Gabai et al., 2023) and is an essential requirement in case of adjuvant and preventive treatments
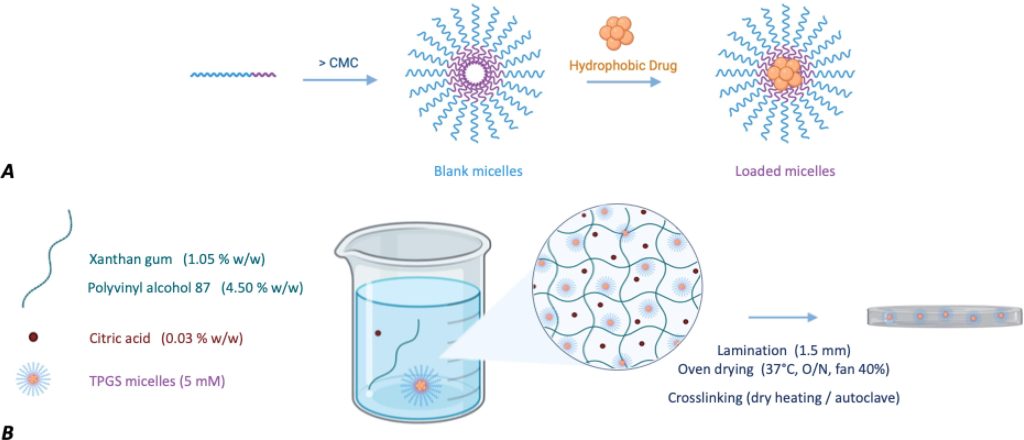
In recent decades, nanometer-sized systems have attracted great interest for ocular delivery (Madni et al., 2017, Qi et al., 2023) and, among them, polymeric micelles have emerged as a promising carrier for the delivery of hydrophobic compounds (Ghezzi et al., 2021). Polymeric micelles (10–200 nm) are based on amphiphilic molecules capable of self-assembling into organized core–shell/supramolecular structures in aqueous media at concentrations exceeding their critical micellar concentrations (CMC). These nanocarriers consist of an inner lipophilic core, which is involved in the loading of hydrophobic compounds, and an outer hydrophilic shell facing the aqueous medium, which is responsible for the interaction of the micelles with the environment (Fig. 1, Panel A). Their capability for overcoming ocular barriers and improving the delivery of lipophilic drugs to the anterior and posterior eye segments has recently been highlighted (Durgun et al., 2020, Vaneev et al., 2021). Among other suitable amphiphilic polymers, micelles made of D-α-tocopheryl polyethylene glycol 1000 succinate (TPGS) gave notable results (Ghezzi et al., 2022; X. Li et al., 2021; Pescina et al., 2021, Rathod et al., 2021). TPGS is a biocompatible and biodegradable non-ionic surfactant, formed by the esterification reaction between vitamin E succinate and polyethylene glycol, and capable of self-assembling into micelles of about 13 nm in size. Previous studies have demonstrated permeation-enhancing activity in cornea and conjunctiva (Duan et al., 2015, Lu et al., 2024, Ostacolo et al., 2013, Pescina et al., 2021, Pescina et al., 2019, Terreni et al., 2021b, Terreni et al., 2020), the capability to diffuse intact within the scleral tissue and to control drug release through esterase-mediated TPGS hydrolysis (Ghezzi et al., 2022, Grimaudo et al., 2018).
Despite the permeation enhancement results – which could support the development of less invasive administration methods – TPGS micellar solutions are characterized by low viscosity and poor adhesion, which reflect in a short residence time at the application site and, in perspective, in the need for frequent administrations. Frequent administrations reduce adherence to the treatment and cause fluctuations in drug tissues concentration with possible side effects.
Therefore, the idea behind this work was the preparation and characterization of a polymeric platform, in the form of a film, that contains micelles (Fig. 1, Panel B) and is capable of controlling their release after ocular administration. The drug-loaded micelles, once slowly released from the platform, can take advantage of their nanometric size, solubilization capability and tissue penetration to enhance drug delivery to the ocular tissues. A further objective of the work was to prepare this platform using biocompatible and sustainable materials, through an easy and scalable preparation process, also paying attention on the sterilization step, a key process for all ocular formulations.
This work relied on the hypothesis that xantham gum (XG), a safe anionic polysaccharide used in swelling-controlled drug release formulations (Chandra et al., 2022, Cortes et al., 2020, Pahuja et al., 2012), can regulate the diffusion of the micelles towards the ocular surface if the pore size of the hydrogel network is adequately tuned. XG is composed of a β-(1 → 4)-D-glucopyranose glucan backbone with side chains of (1 → 3)-α-D-mannopyranose-(2 → 1)-β-D-glucuronic acid-(4 → 1)-β-D-mannopyranose on alternating residues. Compared to other anionic polysaccharides, the branched structure of XG makes it advantageous in terms of mucoadhesivess and structural agent since the conformation of its chains, and thus the viscosity, is less dependent on changes of solvents, temperature or pH (Nsengiyumva and Alexandridis, 2022). Moreover its biotechnological origin confers XG production more eco-sustainable. The film forming capability of XG was reinforced by adding polyvinyl alcohol (PVA), which forms transparent, thin and easily handled films (Cascone et al., 1995, Kodavaty, 2022). XG and PVA have already been associated in a variety of fields, from the food area to the pharmaceutical one, resulting in a promising basis for the preparation of drug delivery systems (Bhattacharya et al., 2012, Bhunia et al., 2013, Brunchi et al., 2016, Ray et al., 2010). TPGS was used for the preparation of the micelles and citric acid (CA) was chosen as the crosslinking agent, to control micelles release. The hydrophobic compounds cyclosporine A (CYC) and melatonin (MEL) were selected as neuroprotective agents to be loaded into the micelles. MEL and several of its metabolites act as free radical scavengers and efficient antioxidants (Hardeland and Pandi-Perumal, 2005, Lundmark et al., 2007, Yu et al., 2021); it also has anti-inflammatory and immunomodulation activity, contributing to the protection of the retinal tissue (Diéguez et al., 2020, Ferreira de Melo et al., 2020, Sande et al., 2014). CYC is widely effective in the treatment of ocular inflammatory states and is also reported to promote retinal ganglion cell survival (Kim et al., 2014). Both MEL and CYC proved to be protective against both hypoxia and glutamate-induced neuronal cell damage (Ruiz et al., 2000, Schnichels et al., 2021; C. Wang et al., 2022; Zhang et al., 2023).
After the definition of film composition, the crosslinking conditions (heating time and temperature) were studied and optimized to control the swelling properties and thus tune the release of the micelles. The ocular biocompatibility was evaluated by HET-CAM assay, while ex vivo studies on isolated ocular tissues were carried out using diffusion cells and two-photon microscopy to confirm the potential of the formulated platform and gain an insight into the penetration mechanism of micelles. Finally, the sterilization step was addressed, and a process was developed to simultaneously crosslink and sterilize the platform.
Download the full article as PDF here A sterilizable platform based on crosslinked xanthan gum for controlled-release of polymeric micelles
or read it here
Materials
Polyvinyl alcohol (PVA, MW 83,400 Da; hydrolysis degree 86.5 – 89.0 mol %) was purchased from Gohsei (Osaka, Japan). Xanthan gum from Xanthomonas campestris (XG, MW 2,000 – 20,000 kDa; 1 % solution Brookfield viscosity 915 cPs; 50 % terminal mannose residues were 4,6-pyruvated while most of the inner mannose residues were 6-acetylated), melatonin (MEL, MW 232.28 g/mol; LogP 1.6) and Nile red (NR, 9-(diethylamino)-5H-benzo[a]henoxazine-5-one; MW 318.4 g/mol) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Tocopheryl polyethylene glycol 1000 succinate (TPGS) was a kind gift from PMC ISOCHEM (Vert-Le-Petit, France). Citric acid (CA) was purchased from A.C.E.F. S.p.a (Fiorenzuola d’Arda, Italy), while cyclosporine A (CYC, MW 1202.6 g/mol; LogP 3 (El Tayar et al., 1993)) was from Alfa Aesar (Haverhill, MA, USA).
Sara Signorini, Andrea Delledonne, Silvia Pescina, Annalisa Bianchera, Cristina Sissa, Maria Vivero-Lopez, Carmen Alvarez-Lorenzo, Patrizia Santi, Cristina Padula, Sara Nicoli, A sterilizable platform based on crosslinked xanthan gum for controlled-release of polymeric micelles: Ocular application for the delivery of neuroprotective compounds to the posterior eye segment, International Journal of Pharmaceutics, 2024, 124141, ISSN 0378-5173, https://doi.org/10.1016/j.ijpharm.2024.124141.

