Rethinking carbamazepine oral delivery using polymer-lipid hybrid nanoparticles
Epilepsy is the most common chronic brain disorder and affects millions of people worldwide. Carbamazepine (CBZ) is one of the first-line pharmacological therapy instituted to patients due to its wide spectrum of action. Although marketed for more than 30 years, CBZ efficacy is strongly limited by its lateral effects, and variable and slow intestinal absorption. This reality leads to resistance drug development, which compromises the patients’ quality of life. Rethinking about CBZ oral delivery is required to achieve a more effective strategy for the treatment of epilepsy.
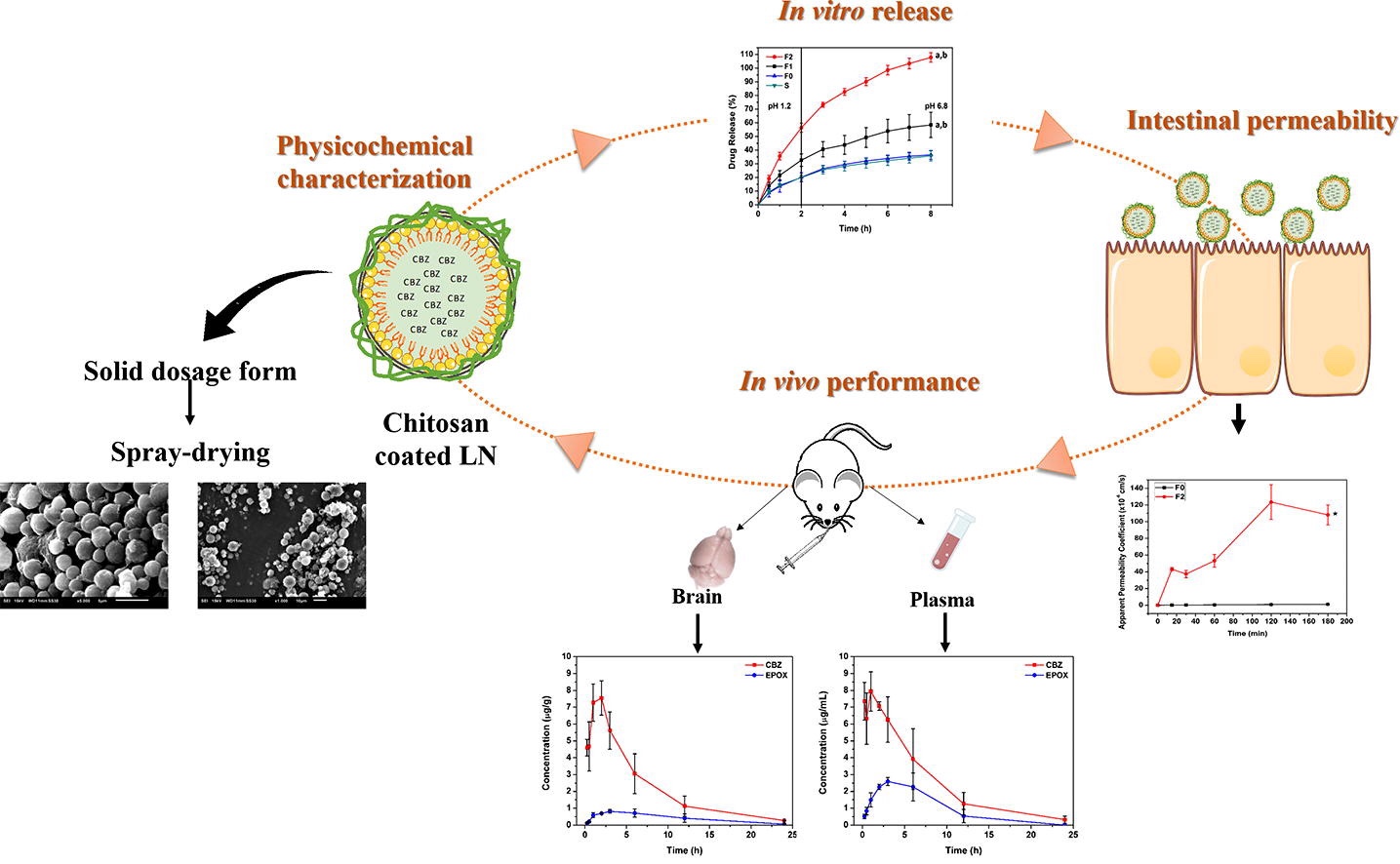
This work aimed at developing polymer-lipid hybrid nanoparticles, using chitosan as the polymer coating, as a surrogate nanosystem for oral administration of CBZ. The hybrid nanoparticles were produced by high-pressure homogenization, and a systematic evaluation was carried out in terms of particle size, zeta potential, loading properties, mucoadhesion, stability and in vitro – in vivo performance assessment, including release, intestinal permeability, and biodistribution studies. Subsequently, nanoparticles were spray-dried and tableted to provide a solid differentiated dosage form.
Chitosan coating improved lipid nanoparticle stability under gastric environment, presenting a size of ca. 150 nm and a polydispersity index smaller than 0.150, and increasing up to two orders of magnitude the drug intestinal permeability.
The in vivo studies herein performed evidenced that hybrid nanoparticles prompted a similar brain disposition of CBZ to that of an oral suspension, after the administration of a lower dose. These findings are consistent with a controlled and reproducible release pattern, suggesting that the therapeutic effect is ensured with lower adverse effects.
The present work may provide a proof-of-concept for further preclinical studies in the treatment of epilepsy.

