Relative Sweetness: How Sweet is Sweet?
Broadly speaking, there are three categories of sweeteners – nutritive, sugar alcohols, and high intensity. Sweetener selection is informed by technical, regulatory and clinical considerations as described in the Introduction to Sweeteners.

A formulator has many choices in selecting a sweetener, but these are not interchangeable, as each has a different sweetness profile – a characteristic intensity, onset, and duration. Understanding the difference between candidates is critical to effective taste masking.
Sweetener Types
The class called nutritive sweeteners consists of roughly a dozen different mono, di, and oligo saccharides commonly used to sweeten foods. These include dextrose, fructose, and (most importantly) sucrose. All have a pleasant sweetness, but are high in calories, cariogenic, and bulky (used in oral formulations at concentrations between 10% and 60%).
For decades, the food industry has endeavored to reduce nutritive sweeteners through the use of low or no-calorie substitutes. Sugar alcohols are one option. These sweeteners are found naturally in small amounts in foods but prepared commercially via the hydrogenation of nutritive sugars or by fermentation. Sugar alcohols do not have the caloric or cariogenic concerns of nutritive sweeteners, but are similarly bulky, taking up 10-60% of the formulation.
More potent are high-intensity, artificial sweeteners. To date, six high-intensity sweeteners are FDA-approved ingredients under the Federal Food, Drug, and Cosmetic Act:
- Saccharin
- Acesulfame potassium
- Aspartame
- Sucralose
- Neotame
- Advantame
With the exception of advantame (approved in 2014), all are precedented for use in drug products per the FDA Inactive Ingredient Database (IID).
Relative Sweetness
The relative sweetness intensity of sweeteners is commonly reported in the literature (Figure 1), but this number is a gross oversimplification.
Figure 1 – Relative Sweetness of Common Sweeteners
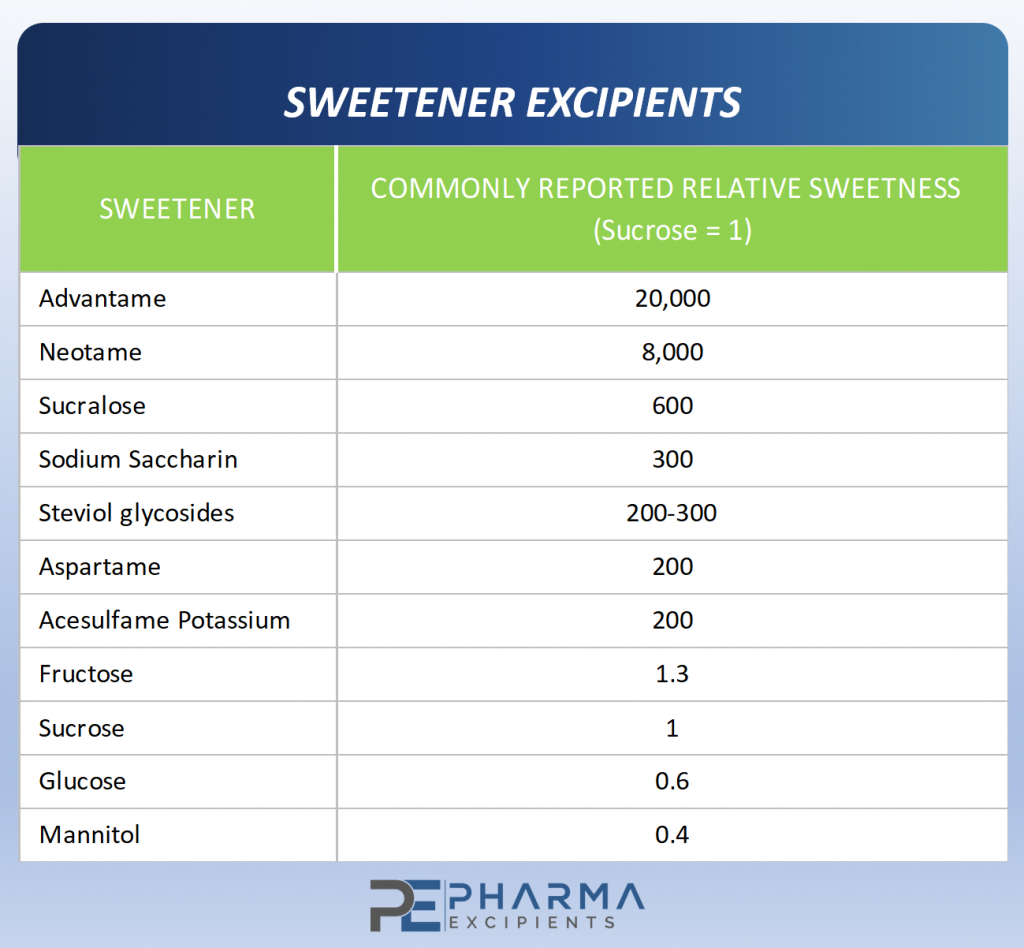 For example, this data suggests that the sweetness of a regular sugar-sweetened cola could be achieved with acesulfame potassium (Ace-K) at 1/200th the concentration of sucrose. In actuality, Ace-K cannot replicate the sweetness of sugar-sweetened cola at any concentration. This is due to differences in sweetness response between sucrose and other sweeteners.
For example, this data suggests that the sweetness of a regular sugar-sweetened cola could be achieved with acesulfame potassium (Ace-K) at 1/200th the concentration of sucrose. In actuality, Ace-K cannot replicate the sweetness of sugar-sweetened cola at any concentration. This is due to differences in sweetness response between sucrose and other sweeteners.
Sweetener Concentration Response
A single number that describes relative sweetness is only appropriate when the concentration-response relationship is constant. This is the case for nutritive sweeteners and sugar alcohols, as they have a linear concentration-response function as shown for sucrose in Figure 2 below. In other words, perceived sweetness increases linearly as sucrose concentration increases. Mathematically, this is describing the slope of the concentration-response relationship.
In contrast, high intensity sweeteners have a nonlinear concentration-response function as shown in Figure 2 for three common high intensity sweeteners. At low concentrations perceived sweetness increases rapidly but then begins to diminish and plateau. In these instances, a single number describing this complex relationship is wholly inadequate.
The underlying cause for this plateau effect is not fully understood. Part of this observed effect may be that the marginal contribution of sweetness intensity is decreasing with increasing concentration. Another potential explanation is that above a certain concentration, artificial sweeteners begin to taste bitter. This emerging bitterness self-balances with the marginal sweetness, resulting in the observed plateau.
From a formulation standpoint, the location of where this sweetness plateau ultimately occurs is extremely important. For some sweeteners this is well below the upper limit of perception of sucrose. For example, a typical sugar-sweetened soft drink has a sweetness intensity of about 2½ on the Flavor Profile intensity scale. Ace-K has a maximum sweetness intensity of about 2. For this reason, Ace-K cannot be used alone in zero-calorie soft drinks, but rather must be used in combination with other high-intensity sweeteners such as aspartame or sucralose.
In summary, relative sweetness data is meaningless in the absence of the corresponding sucrose concentration. These values are even of less value to pharmaceutical scientists as the required sweetness for most bitter APIs is well above food applications for which the data was derived.
Figure 2. Concentration Response Curves for Selected Sweeteners 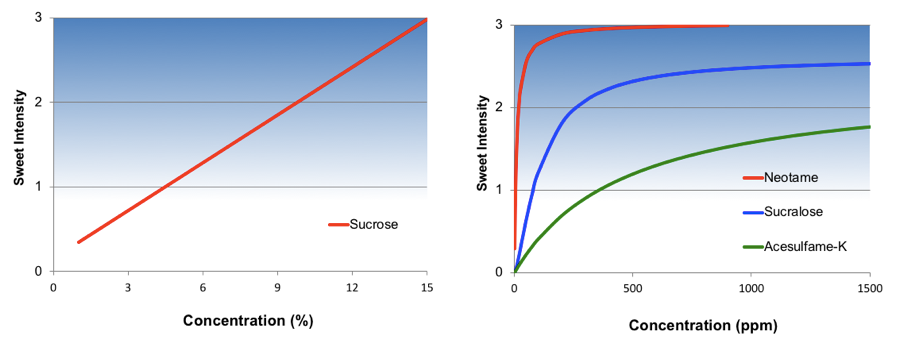
Source: Dubois 1990 (Adapted)
Understanding Sweeteners
When faced with an intensely bitter API, a formulator may choose to start with the most potent sweetener candidates. However, as described, the values reported in the literature can be misleading.
In working with high intensity sweeteners, if a little is good, more is not better – most artificial sweeteners become bitter at inappropriately high usage levels.
Every sweetener has a different sweetness profile – a characteristic intensity, onset, and duration. Relative sweetness intensity is an important but insufficient consideration in selecting sweeteners for palatability and taste masking. Other important characteristics of sweeteners are the onset and duration of sweetness, which will be discussed in a separate post.
Blog Post for pharmaexcipients.com – Prepared by Senopsys LLC by David Tisi — All Rights Reserved
David Tisi is the Technical Director of Senopsys LLC, a specialty services firm dedicated to the development of palatable drug products. He has 15 years of experience in taste assessment and taste masking of investigational and approved drugs for children and adults, leveraging his background in sensory science and food chemistry. He can be reached at [email protected]

