Development and application of control concepts for twin-screw wet granulation in the ConsiGma-25: Part 2 granule size
Abstract
Traditional operation modes, such as running the production processes at constant process settings or within a narrow design space, do not fully exploit the advantages of continuous pharmaceutical manufacturing. Integrating Quality by Control (QbC) algorithms as a standard component of production processes can mitigate the effect of diverse process disturbances and enhance process efficiency, particularly in terms of production costs and environmental footprint. This paper explores the potential of QbC algorithms for optimizing twin-screw wet granulation in the ConsiGmaTM-25 manufacturing line, specifically addressing granule size. It represents the second part of a study (Celikovic et al., 2024) focused on granule composition.
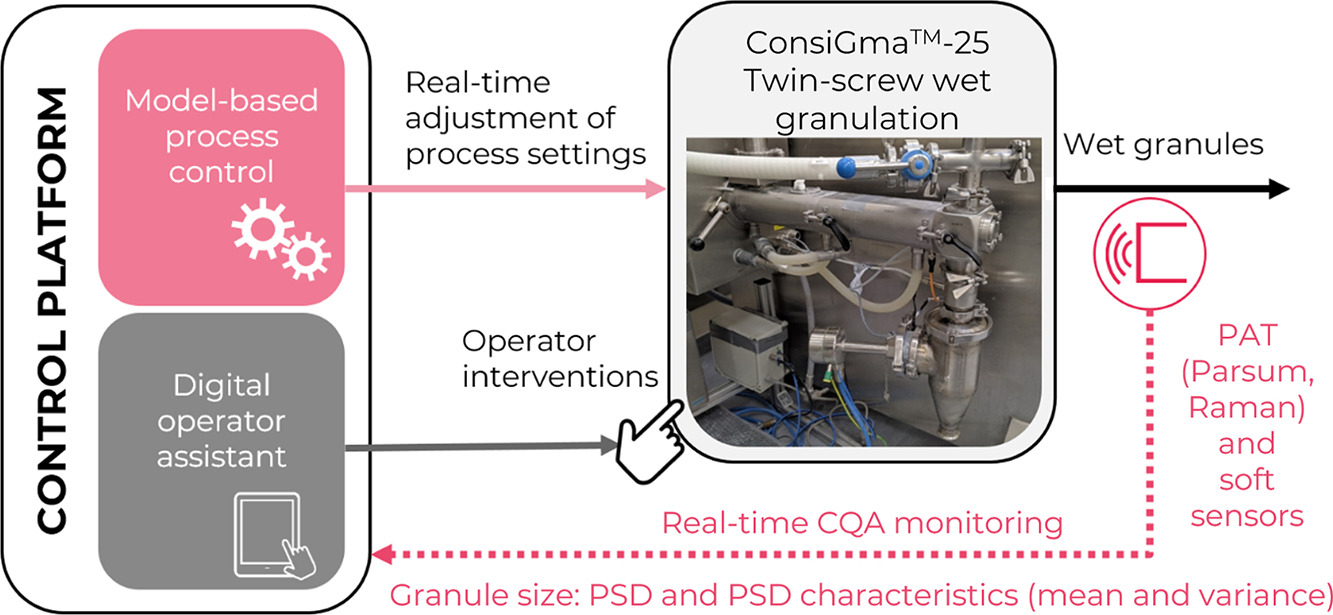
The concepts proposed in this work rely on process analytical technology (PAT) equipment for real-time monitoring of the granulation CQAs and a dynamic process model linking the granulation process parameters and the monitored CQAs. The granule size model identified via the local-linear-model-tree (LoLiMoT) algorithm is used to develop both a model predictive controller (MPC) and a granule size soft sensor. The MPC employs this model as a core component for selecting optimal granulation parameters to ensure the production of granules with target size. A digital operator assistant is developed to address disturbances that cannot be mitigated via MPC but can be eliminated by the plant operators.
This study systematically outlines a workflow, starting from conceptualization, moving through simulation development, and finally ending with real-world application on a production line. In this final step, all proposed concepts are transferred to the ConsiGmaTM-25 manufacturing line, where their performance is validated through selected disturbance scenarios.
Introduction
A conventional approach to ensuring the safety and quality of pharmaceutical products is the so-called Quality-by-Testing (QbT) principle. QbT relies on offline laboratory analysis while neglecting the information collected during the manufacturing phase. In contrast, Quality-by-Design (QbD) aims to ensure product quality by understanding and managing the relationships between material attributes, process parameters, and product quality attributes (Zhang and Mao, 2017). QbD relies on inline acquired data, e.g., collected via process analytical technology (PAT) tools, and allows more sophisticated process and quality control options. The Quality by Control (QbC) builds upon the QbD principle by focusing on advanced process control techniques. QbC advances systematic quality assurance in pharmaceutical manufacturing and facilitates the transition to next-generation smart manufacturing, commonly known as Industry 4.0 (Su et al., 2019). To exploit the advantages offered by continuous manufacturing of pharmaceuticals, it is essential to adopt QbC methodologies as a standard component within traditional production facilities. Hence, the transition from the conventional mode of running processes (i.e., at constant settings or within a narrow design space, usually accompanied by minimal process monitoring or disturbance mitigation measures) to QbC approaches is crucial. The QbC approaches involve control algorithms that ensure maintaining target product characteristics through real-time adjustment of process parameters (process control) or removing out-of-specification intermediate products from the production stream (quality control). These control algorithms are enabled by PAT and soft (virtual) sensors (i.e., the models or algorithms used to predict a quality attribute using the process or other available sensor data) and reinforced by operator support algorithms (i.e., the algorithms providing real-time assistance or instructions for plant operators). The paper at hand proposes and explores the potential of such QbC algorithms, with a focus on the granule size after the twin-screw wet granulation unit in the industrial ConsiGmaTM-25 manufacturing line (GEA, 2023).
Twin-screw wet granulation is a common pharmaceutical process used to improve powder characteristics, such as homogeneity and flowability. This granulation process takes place inside a twin-screw granulator, where twin screws and granulation liquid support the agglomeration of powder particles, resulting in the formation of wet granules. The resulting particle size distribution (PSD) of wet granules is recognized as an intermediate critical quality attribute (CQA) directly affecting the final product quality, e.g., tablet dissolution characteristics (Markl et al., 2020, Zaborenko et al., 2019). The creation of out-of-specification (OOS) PSD can cause several undesired events, e.g., clogging of the dryer filters due to extensive formation of fine particles or blockages of transfer lines and following unit operations due to agglomerate formation. Thus, producing wet granules with well-defined PSD is critical for achieving constant product quality and production robustness. This manuscript focuses on granule size and expands upon the study presented in Celikovic et al. (2024), which investigated granule composition. Intermediate CQAs, such as granule density or flowability, are not investigated in these studies.
The interest in continuous wet granulation, specifically in the field of exploring and modeling co-dependencies between the critical process parameters (CPP) and CQAs, has significantly grown over the last decade (Wang et al., 2022, Barrasso et al., 2013, Barrasso et al., 2014, Metta et al., 2019). However, a common limitation of existing modeling approaches is high computation time, which limits their applicability for process control purposes. Shirazian et al. (2017) underlines this limitation and proposes an alternative data-driven modeling approach based on artificial neural networks (ANN). The proposed model exhibits significantly reduced computation time and confirms the potential of the chosen modeling approach. However, the resulting nonlinear ANN relation is a static one. Hence, it would not represent a sufficient basis for the design of control concepts optimizing the dynamic process behavior, such as a model predictive controller (MPC). The significance of real-time granule size process control was also recognized and addressed in Nicolaï (2019). This research proposes solutions for real-time process monitoring of granule size and outlines the challenges related to process modeling and control. Furthermore, it raises a question of the non-linear behavior, which would compromise the performance of standard linear control concepts (e.g., of the designed PID controller) in the investigated process. The study by Celikovic et al. (2023) addresses this challenge by proposing a dynamic non-linear process model that links the granulation CPPs and PSD characteristics based on the local-linear-model-tree (LoLiMoT) approach Nelles (1997). LoLiMoT is a data-driven algorithm that combines decision tree principles with locally weighted linear regression. The tree structure and linear regression parameters are optimized during the training to achieve satisfactory agreement between the training data and model prediction. Each leaf node of the resulting tree structure contains a linear regression model relevant to a specific region of the operating space. LoLiMoT is suitable for capturing dynamic, complex, and non-linear relations while maintaining the beneficial properties required for process control. Like this, the proposed model represents a necessary basis for developing model-based control concepts (e.g., MPC) in this work.
Apart from strict administrative regulations that are not the focus of this manuscript, a fundamental challenge decelerating the application of control concepts in the pharmaceutical industry are the limitations regarding reliable PAT equipment and the effort required to identify the process models. Relying on previously established solutions for real-time intermediate CQA monitoring and process models linking relevant CPPs and intermediate CQAs (Celikovic et al., 2023), we now introduce a control platform for the twin screw granulation unit in the ConsiGmaTM-25 manufacturing line. The proposed control platform aims to ensure the production of wet granules with a well-defined PSD. The platform integrates a LoLiMoT-based MPC for real-time process control of the granule size and a digital operator assistant supported by a fault detection algorithm. Furthermore, an alternative soft sensor for granule size relying on the LoLiMoT-based process model and Raman-based PAT probe is proposed. The proposed algorithms are developed in a simulation environment and are then transferred to the ConsiGmaTM-25 manufacturing line. Finally, the performance is demonstrated in the real system through selected disturbance scenarios. This manuscript provides a systematic workflow covering conceptualization, simulation development, and real-system application and can be used as a step-by-step guide for the implementation of the introduced control platform in a fast and efficient manner.
Read more here
Materials
The investigated pre-blend contained methyl 4-hydroxybenzoate (Sigma Aldrich, USA) as the API surrogate material, VIVAPHARM HPMC E5 (Demacsa, Mexico), Avicel PH101 (DuPont, Ireland) and Granulac 200 (Meggle, Germany) as the excipients. Deionized water was used as granulation liquid.
Selma Celikovic, Johannes Poms, Johannes Khinast, Martin Horn, Jakob Rehrl, Development and application of control concepts for twin-screw wet granulation in the ConsiGmaTM-25: Part 2 granule size, International Journal of Pharmaceutics, 2024, 124125, ISSN 0378-5173, https://doi.org/10.1016/j.ijpharm.2024.124125.
See our next webinar:
“Rethinking the development of controlled release formulations and manufacturing processes”
Date: 30th of April, Time: 3:00 pm (Amsterdam, Berlin)


