Advances in Nanocarrier Systems for Overcoming Formulation Challenges of Curcumin: Current Insights
Abstract
Introduction
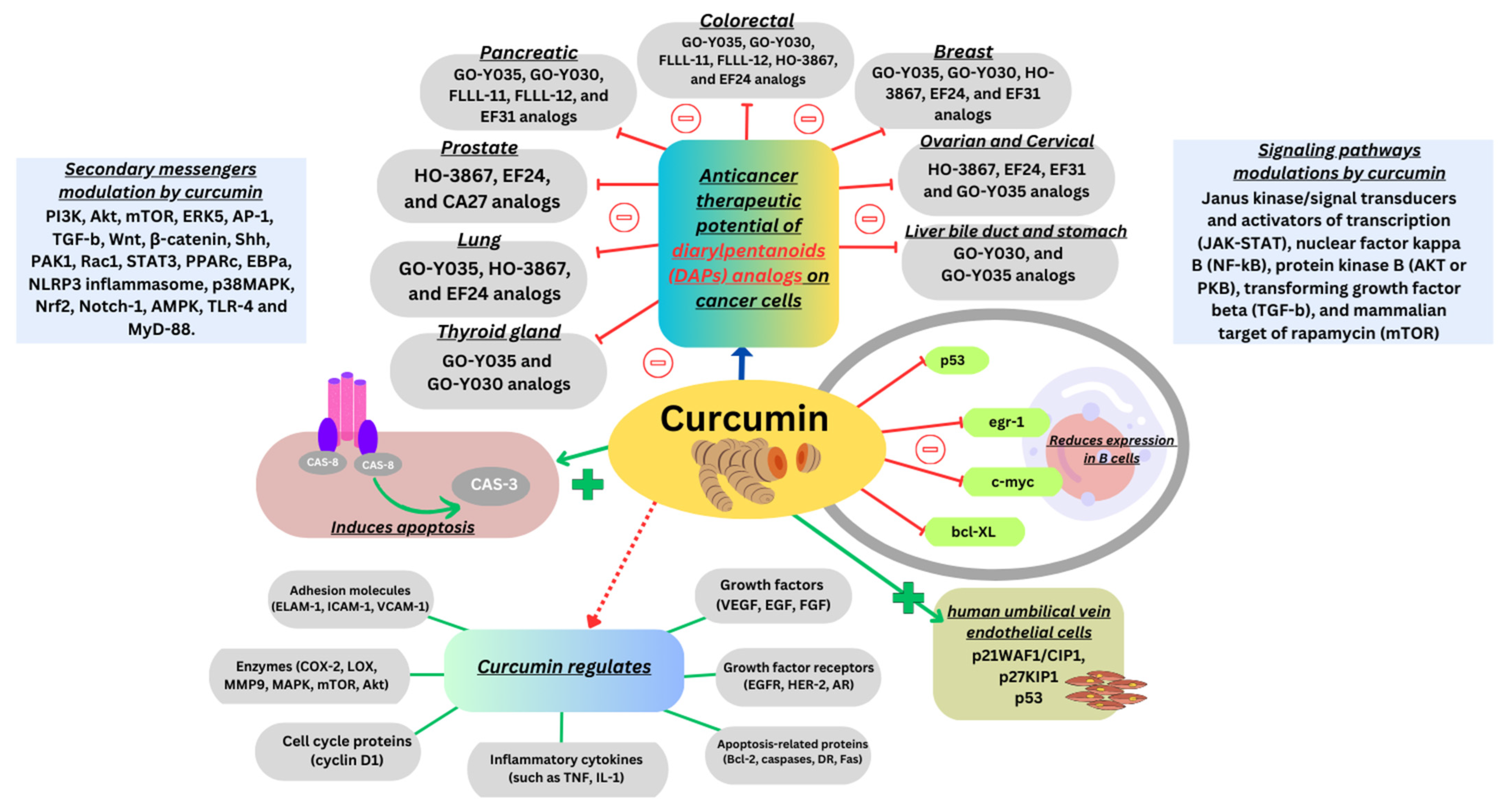
Curcumin is a primary constituent found in the rhizome of Curcuma longa (L.) and other Curcuma spp., commonly known as turmeric, which belongs to the Zingiberaceae family. Turmeric mainly consists of water (80–90%), with carbohydrates accounting for almost 13%, proteins 2%, minerals 2%, and lipids accounting for less than 1% [1]. Curcuminoids, the important compound in turmeric account for 10% of the total dry powder. Curcuminoids primarily consist of curcumin, accompanied by two other diarylheptanoids referred to as desmethoxycurcumin and bis-demethoxycurcumin. The World Health Organization specified a permissible daily intake for curcumin as a food additive up to 3 mg/kg of body weight. Curcumin, being a hydrophobic polyphenol, exhibits a variety of biological and pharmacological effects. Curcuminoids have acquired extensive recognition for their widely reported attributes, encompassing their ability to act as antioxidants, reduce inflammation, and combat microorganisms, in addition to inhibiting the growth of cancer [2,3]. Additionally, research has shown promise in curcumin’s ability to regulate blood sugar levels, offering potential benefits for diabetes management [4]. The potential advantages of curcumin for Alzheimer’s disease [5], atherosclerosis prevention and the management of neoplastic diseases, particularly those affecting the digestive tract, are gaining attention [6]. Its consistent immunomodulatory effects have been demonstrated [7] and have shown notable efficacy in preventing osteoporosis [8], cardiovascular ailments [9], obesity [10] and liver diseases [11]. These findings collectively underscore curcumin’s versatile and promising applications in diverse health conditions. Furthermore, investigations have demonstrated that curcumin influences various cell signaling pathways, enhances the expression of p53, p21, and p27, reduces the levels of gene products promoting cell survival, and triggers apoptosis [12]. Several clinical trials have confirmed its remarkable safety, tolerability, and efficacy, even when administered at elevated oral doses. As a result, it is currently available as a dietary supplement globally [13]. The efficiency of the combined therapy of curcumin with chemotherapeutic agents was reviewed recently [14].
The current review employs a systematic methodology, conducting thorough literature searches across various online databases using key terms such as curcumin, curcumin delivery, nanoparticles, physicochemical properties, molecular pathways, pharmacokinetics, liposomes, nanoemulsions (NEs), polymeric nanoparticles (PNs), polymeric micelles (PMs), dendrimers, solid lipid nanoparticles (SLNs), nanostructured lipid carriers (NLCs), clinical trials, patents, and evaluation. Employing a systematic methodology, the review incorporated structured data extraction, ensuring a methodical and organized approach to gathering pertinent information from diverse sources. The compilation of this information was conducted with precision, weaving together a cohesive narrative that encapsulates the current advancements and emerging trends in curcumin drug delivery. Notably, this approach was tailored to accommodate the distinctive functionalities and intricacies inherent in each database, enhancing the reliability and robustness of the review’s findings.
Download the full article as PDF here Advances in Nanocarrier Systems for Overcoming Formulation Challenges of Curcumin
or read it here
Jacob, S.; Kather, F.S.; Morsy, M.A.; Boddu, S.H.S.; Attimarad, M.; Shah, J.; Shinu, P.; Nair, A.B. Advances in Nanocarrier Systems for Overcoming Formulation Challenges of Curcumin: Current Insights. Nanomaterials 2024, 14, 672. https://doi.org/10.3390/nano14080672
See our next webinar:
“Rethinking the development of controlled release formulations and manufacturing processes”
Date: 30th of April, Time: 3:00 pm (Amsterdam, Berlin)


