Polymeric microspheres redefining the landscape of colon-targeted delivery: A contemporary update
Abstract
During recent times, the delivery of the medications to the colon has seen more interest by the researchers, as it proved to be providing both options for treating local colon-related conditions and a route for systemic delivery of the various other types of medications. For these to happen, the medication has to provide protection from severe conditions in the stomach and small bowel, which either degrade the medication or may cause its premature release and uptake in the upper part of the digestive track. This review describes the various roles of microspheres as a colon-targeted drug delivery device (CTDDD). Through these review, we try to provide thorough information about the effects of the physiology of the colon. Also, we made an effort to highlight different mechanisms of colon targeting. Along with these, we have pointed out some of the important evaluation factors for carrying out a thorough investigation about the physicochemical and pharmaceutical properties of microspheres for targeting the colon. Also, we exchange views about the applications of microspheres as CTDDD in different diseases and disorders of the colon. Plus, we discuss the different challenges that occur during the formulation and targeting of these microspheres. At last, we share our thoughts on the possibilities in the near future in these domains, which will help in changing the scenario of how we can treat colon-related problems.
Introduction
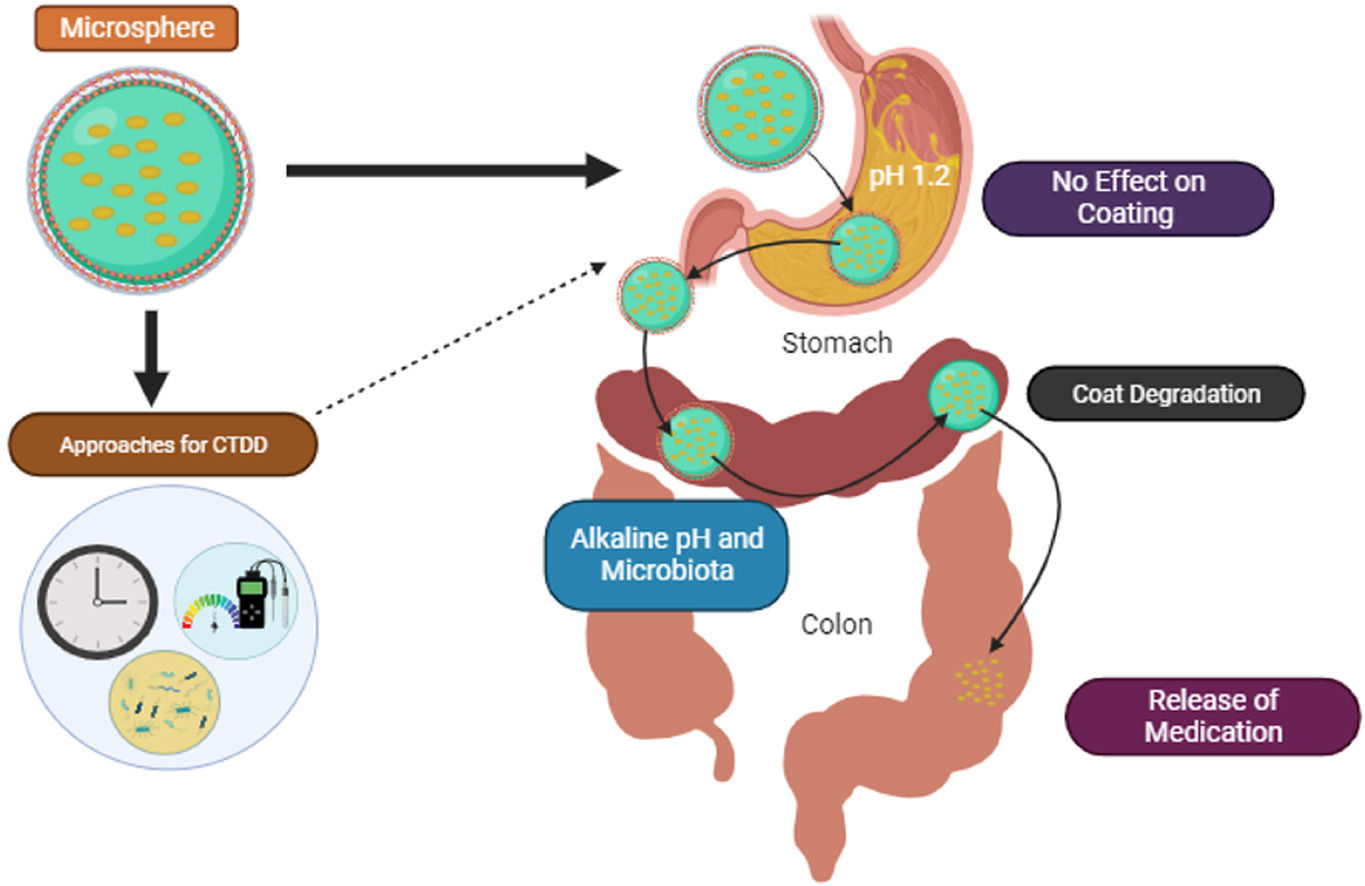
Colonic diseases are seen to be widely spreading, which obviously demands the development of adroit, targeted treatments to improve the safety and effectiveness of medication therapy [1,2]. Colorectal carcinoma (CRC) is reported as one of the three most rampant causes of cancer-related deaths on the planet, which brings about over 850,000 people to lose their lives annually [3]. Additionally, chronic inflammatory bowel diseases (CIBD) are seen to be more frequent in regions like Asia [4]. Therefore, colonic disease prevention is crucial for global public health. Colon-targeted drug delivery devices (CTDDD) are being sought after to address these concerns. These devices aim to inhibit release and uptake from the upper digestive tract while releasing medications in response to the colonic environment [5]. Non-targeted medicines may cause adverse reactions and lower efficacy due to systemic absorption before reaching the colon. Apart from that, colon-targeted drug delivery devices provide a technique to increase the bioavailability of medications, specifically large biomolecules like proteins and peptides, which are susceptible to breakdown in the acidic and enzymatic conditions of the upper gastrointestinal tract (GIT) [6]. Given its lesser variability and frequency of digesting enzymes than the small intestine, it has been presumed that the colonic part of the GIT provides an ideal place to absorb protein or polypeptide medicines. CTDDD prevents peptide medication hydrolysis along with breakdown by enzymes throughout the duodenum as well as the jejunum and allows release when they reach the ileum or colon, which will eventually lead to higher systemic bioavailability. Additionally, the colon can be susceptible to enhancers of absorption due to the extended residency period (a maximum of five days) [7]. Rectal delivery offers a quicker way to deliver medications to the colon than oral administration, yet it might be challenging to make it to the proximal colon and discomforting for those taking medications. Drug preparations for intra-rectal usage are available in a variety of types, including solutions, foam, and suppositories, to treat the large intestine both systemically and topically. Drug concentration is influenced by formulation variables, retrograde spreading, in addition to retention time. Topical application is principally responsible for the effectiveness of medications absorbed into the colon. Notably, enema solutions have a greater spreading capacity than foam and suppositories, which are primarily held in the rectum and sigmoid colon [8].
For the successful creation of CTDDD, both the changed microenvironment close to disease sites and the special physiological properties of the colon must be taken into account. Throughout the stomach and towards the intestine, the GIT undergoes dynamic fluctuations in pH, enzymatic levels, fluid matter, and motility. Additionally, the colon microenvironment near illness sites is very different from that in healthy areas. All individuals with colonic problems have injuries to the mucosa, elevated reactive oxygen species and inflammatory cytokine levels, and an unbalanced level of vital antioxidants in their bodies. Numerous formulation techniques have been investigated to enhance colonic medication delivery in light of these pathophysiological alterations. These consist of systems that are pH-sensitive, enzyme-activated, and magnetically-driven [[9], [10], [11]]. Receptor-mediated systems have also been researched with the aim of interacting selectively with particular receptors highly expressed around the site(s) of the disease. Microspheres can be made into variable sizes (between the scales of micrometers and millimeters). With their minute size, which leads to a greater surface area, microspheres have an upper hand in medication delivery as they bring forth the release of the medication in a controlled release profile by entrapping the medication as a perfect formulation for site-specific targeting in the large intestine [[12], [13], [14], [15], [16]]. Microspheres, designed as CTDDD, are formulated using pH-dependent and/or enzyme-responsive polymers, which prevent the medication from being released inside the stomach or even small intestinal regions and release the medication in colonic conditions by undergoing either pH-succeptible degradation or enzymatic degradation. Along with that, microspheres are coated with various pH-dependent polymeric coatings to retard medication release in the upper part of the GIT [17]. By means of this review, we want to provide an overview of the microsphere formulation considerations and methods, along with the influence of the colon’s physiology over it [18]. These microspheres can be formulated to target the large intestine, utilizing the diverse types of approaches to targeting the large intestine [19,20]. Additionally, we discuss the challenges, recent trends, and possible developments in this domain in the near future [21].
Table 1 Recent advancements in CTDD via microspheres
| Fabrication Technique | Polymer | Medication | Size (μm) | Entrapment Efficiency | Mechanism of Medication release | Uses/Treatment | Study type |
|---|---|---|---|---|---|---|---|
| Resistant Starch | Aspirin | – | 68.96 | Enzymatic degradation | CIBD | In vitro | |
| Eudragit S100 | Lactobacillus rhamnosus GG | 5.2–7.3 | – | pH susceptible degradation | Probiotics therapy | In vitro | |
| Eudragit S-100 | Mesalamine | 4.91 | 7 ± 0.89 | pH susceptible degradation | UC | In vitro | |
| Chitosan | Curcumin overloaded with ascorbic acid | – | 91.2 ± 0.88 | pH susceptible degradation | CRC | In vivo | |
| Inulin | Mesalamine | 0.8–10 | 87 | Enzymatic degradation | UC | In vitro | |
| Polyacrylamide-graft-gum karaya | Capecitabine | 1.02–8.19 | 77.30–88.74 | pH susceptible degradation | CRC | In vitro | |
| Chitosan | Meloxicam | – | 65.5 ± 1.5–84.1 ± 1.7 | pH susceptible degradation | CRC | In vivo | |
| Eudragit S-100 | 5-Fluorouracil | – | 99 | pH susceptible degradation | CRC | In vivo | |
| Zein (ZN) and Gantrez® AN119 (PVMMA) | Curcumin | 10.15–25.64 | 89 | pH susceptible degradation | CIBD | Ex vivo | |
| PGA-co-PDL | Indomethacin | – | 63.16 ± 3.5 | pH susceptible degradation | CIBD | In vitro | |
| Polyacrylamidegrafted-CMCNa copolymer | Capecitabine | 1.00–7.34 | 70.98 ± 1.23–94.41 ± 0.45 | pH susceptible degradation | CRC | In vitro | |
| Eudragit® FS 30D | Glutathione and S-nitrosoglutathione | 5 ± 1–7 ± 1 | 74 ± 3–82 ± 2 | pH susceptible degradation | Therapy for Crohns disease | In vitro | |
| Ionic gelation | Inulin/Chitosan/Alginate | Quercetin | 25.1 ± 1.8–79.4 ± 4.5 | 53.2 ± 1.2 | Enzymatic degradation | CIBD | In vitro |
| Sodium alginate | Gallic Acid | – | 11.26–72.64 | Enzymatic degradation | CRC | Ex vivo | |
| Alginate | Astaxanthin | 0.5–3.2 | – | Enzymatic degradation | UC | In vitro | |
| Gum odina - Sodium alginate | Capecitabine | 568.33 ± 45.76 | 45.91 ± 2.94 | Enzymatic degradation | CRC | Ex vivo | |
| Pectin/NaCMC | Progesterone | 1031 ± 19 | 80.1–97.8 % | Enzymatic degradation | Hormone therapy | Ex vivo | |
| Sodium alginate | Meloxicam | 109.16 ± 0.96–1025.12 ± 0.29 | 50.33 ± 0.40 to 74.93 ± 0.69 | Enzymatic degradation | Rheumatoid arthritis management | In vivo | |
| Konjac glucomannan/Sodium alginate/Graphene oxide | Ciprofloxacin | – | 19.11 ± 1.39 | Enzymatic degradation | CIBD | In vitro | |
| Arabinoxylan | Insulin | 150–300 | 71.3 ± 2.7 | Enzymatic degradation | Management of Diabetes | In vitro | |
| Locust bean gum | Mesalamine | 1450 | 32.64 ± 0.57–57.42 ± 1.98 | pH susceptible degradation | UC | In vitro | |
| Portulaca oleracea polysaccharide/Alginate/Borax | 5-fluorouracil | 930–1140 | 53–88 | Enzymatic degradation | CRC | In vitro | |
| Chitosan | Flurbiprofen | 700–1300 | 20.3 ± 0.007–78.8 ± 0.003 | pH susceptible degradation | Non steroidal anti-inflammatory drug (NSAID) | In vitro | |
| Pectin/NaCMC | Progesterone | 1114 ± 36.9–1447 ± 35.7 | 82–99 | Enzymatic degradation | Hormone therapy | Ex vivo | |
| Gelan gum | Ketoprofen | 700.17–938.32 | 48.76 to 87.52 | pH susceptible degradation | NSAID | In vitro | |
| Double emulsion | Methocel E5/Eudragit L100 | Captopril | 110–128 | 95 | pH susceptible degradation | – | In vitro |
| PLGA/PVA | Fucoxanthin | 2.01–10.95 | 33.09–34.87 | pH susceptible degradation | CRC | In vitro | |
| Eudragit® RS100 | Fluorescein isothiocyanate-dextran | 31.1 ± 0.5 | – | pH susceptible degradation | – | Ex vivo | |
| Gum Katira | 5-fluorouracil | – | 79.71 ± 6.01 | pH susceptible degradation | CRC | In vivo/Ex vivo | |
| Eudragit® FS 30D/Eudragit® RS-PO | Enoxaparin sodium | 80.64–165.00 | 62.38–94.89 | pH susceptible degradation | Anticoagulant | In vitro | |
| PLGA/PVA | Fucoxanthin | 3.93–17.12 | – | pH susceptible degradation | CRC | – | |
| Tulsion® Thermocoat L 30 D55 | Clarithromycin | 52.0 ± 0.46 | 61.0 ± 3.1 | pH susceptible degradation | – | In vivo | |
| PLGA | PEGylated apoptotic protein | 14.4 ± 1.8 | 85.7 % ± 4.1 | pH susceptible degradation | CRC | In vivo | |
| Emulsion solvent Evaporation | Cellulose/Alginate | Mesalazine | – | – | pH susceptible degradation | CIBD | In vitro |
| Halloysite nanotube/Chitosan | Paeoniflorin | 26.7 ± 7.5 | – | pH susceptible degradation | UC | In vitro | |
| Eudragit® FS 100 | Curcumin | 98.26 ± 6.38 | pH susceptible degradation | CIBD | In vivo | ||
| Pectin | Lamivudine | – | 30.31–66.32 | Enzymatic degradation | Chronic Hepatitis B | In vitro | |
| Resistant Starch | Ciprofloxacin HCl | 0.743–4.156 | – | pH susceptible degradation | Antimicrobial | In vitro | |
| Pectin/Chitosan | Acetoaminophen | 24.98 ± 13.67 and 0.62 ± 0.24 | 18.86–64.33 | pH susceptible degradation | – | In vitro | |
| Eudragit L100–55 and S100 | Celecoxib | – | 72.67 ± 3.93–84.33 ± 2.12 | pH susceptible degradation | UC | In vivo | |
| Chitosan | Curcumin | 10.94 ± 0.16–24.13 ± 0.62 | 73.88 ± 0.54–83.37 ± 0.62 | pH susceptible degradation | CIBD | In vivo | |
| Chitosan | 5-fluorouracil/Leucovorin | 15 to 35 | 11.6 ± 0.09–21.8 ± 0.12 | pH susceptible degradation | CRC | In vitro | |
| Pectin | Metronidazole | 14.02 ± 1.03 | 80.61 ± 2.42–94.52 ± 2.25 | Enzymatic degradation | Antimicrobial | In vitro | |
| PEG-cross-linked Chitosan | 5-fluorouracil | 316 ± 20 | – | pH susceptible degradation | CRC | In vivo | |
| Coacervation | NaCMC/Pectin | Potassium diformate | – | – | pH susceptible degradation | Bacteriostatic | In vitro |
| Chitosan-Alginate | Quercetin | – | 86.91 ± 1.10–93.11 ± 0.72 | pH susceptible degradation | Antimicrobial | In vitro | |
| Chitosan-Alginate | Ruta graveolens L. Phytocomplex | – | 31.99 ± 0.12–75.31 ± 0.96 | pH susceptible degradation | Antioxidant | In vitro | |
| Agave Fructans | Ibuprofen | – | 0.8–21.5 | Enzymatic degradation | NSAID | In vitro |
Download the full article as PDF here Polymeric microspheres redefining the landscape of colon-targeted delivery
or read it here
Raosaheb S. Shendge, Tejas S. Zalte, Shubhangi B. Khade, Polymeric microspheres redefining the landscape of colon-targeted delivery: A contemporary update, European Journal of Medicinal Chemistry Reports, Volume 11, 2024, 100156, ISSN 2772-4174, https://doi.org/10.1016/j.ejmcr.2024.100156.
See our next webinar:
“Rethinking the development of controlled release formulations and manufacturing processes”
Date: 30th of April, Time: 3:00 pm (Amsterdam, Berlin)


