Pharmaceutical oils for Cannabinoid drug products from ADM-SIO
Medicinal Cannabinoids
There are currently more than 150 cannabinoid-based products under development in the world. The first US FDA approved product Epidiolex®, isolated from the cannabis plant and indicated for severe forms of epilepsy is formulated with Sesame oil. Other approved products like Marinol® are also formulated with oils.
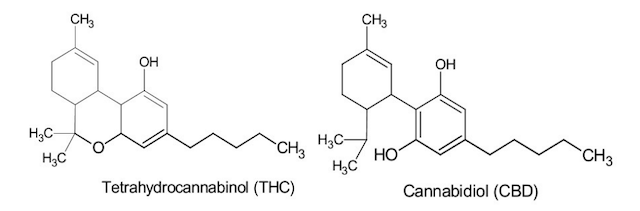
Cannabinoids, generally, have a very low solubility in water and are highly lipophilic. Among the hundreds of phyto-cannabinoids in cannabis, tetrahydrocannabinol (THC) and cannabidiol (CBD) have been studied in many different therapies.
Our portfolio for the formulation of your cannabinoids drug products
- Refined Sesame Oil IV-1
- Refined Olive Oil IV
- Refined Olive Oil PharmaPlus
Our highly purified oils are fully compliant with the European Pharmacopoeia (EP) and United States Pharmacopeia/National Formulary (USP/NF) monographs and general notices intended for use in the manufacturing of pharmaceutical products.
Manufactured under cGMP according to ICH-Q7 Good Manufacturing Practice Guidance for Active Pharmaceutical Ingredients or to the Joint IPEC-PQG Good Manufacturing Practice Guidance for Pharmaceutical Excipients.
Supported with Certificate of Suitability to the European Pharmacopoeia (CEP) granted by the European Directorate for the Quality of Medicines & Healthcare (EDQM) and/or with Drug Master File (DMF) type IV for excipients submitted to the U.S. Food and Drug Administration (FDA).
 Download the full white paper as a PDF here
Download the full white paper as a PDF here


