Ibuprofen DC-100 for direct compression – See the chance for your process simplification
Pharmatrans SANAQ® offers Ibuprofen DC100 as an enhanced powdered active ingredient to assist simplified production of superior oral Ibuprofen formulations.
This improved physical form of Ibuprofen can be incorporated into designed formulations, both
for increasing process simplicity or for enhancing the efficiency of coating and layering properties.
Ibuprofen applications
The need for improved Ibuprofen formulations is driven by the high market demand for this nonsteroidal anti-inflammatory drug (NSAID) for the treatment of pain, particularly for headache, as well as to relieve fever and inflammation. The drug is used in diverse dosage forms, usually with uptake is favored intravenously or orally, by way of tablets, sachets, capsules, or stick packs, with varying drug dissolution profiles ranging from immediate release to controlled release.
Main challenges with Ibuprofen OSD formulations include taste masking and overcoming low compression and compaction properties in tablet manufacturing. Compression of Ibuprofen demands sophisticated treatment of the drug to achieve compressibility. Long compression cycles are critical as the product tends to stick after a while due to the low melting point and especially with using low compression forces leads to manufacturing problems.
Pharmatrans SANAQ® Ibuprofen DC-100 solution
Ibuprofen DC 100 complies with European and American pharmacopeia definitions (Ph. Eur, USPNF) and takes the form of spherically shaped particles consisting of 100 % Ibuprofen drug substance granulated by patented process technologies, with no additional excipients Ibuprofen DC 100 is thus a uniquely directly compressible form of Ibuprofen with a shape and surface structure that provides distinctive product characteristics, also including smooth surface, low porosity, good flow properties, mechanical stability, and uniform particle size distribution in the range of 250-400 μm.
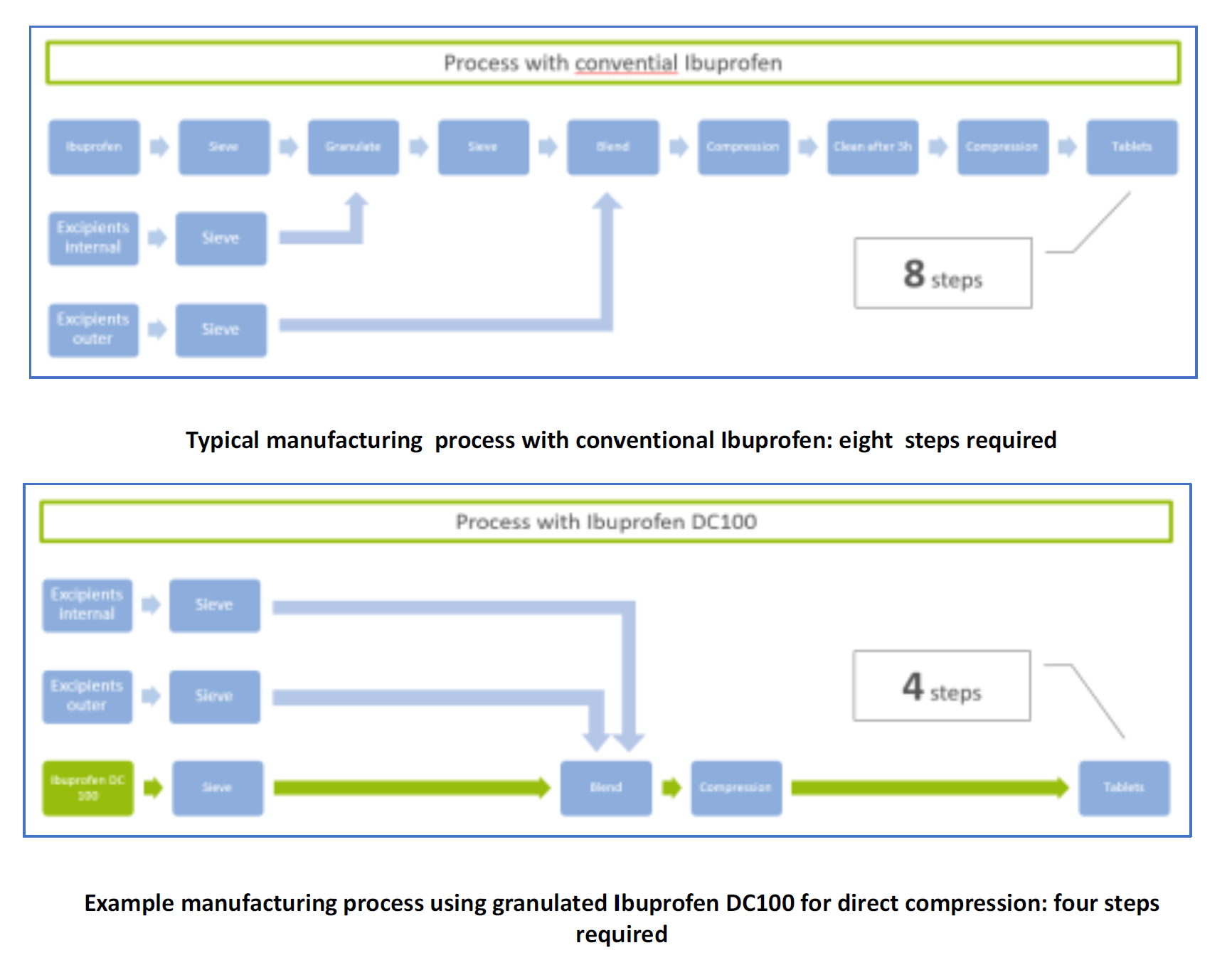
This allows an easy substitution in Ibuprofen formulations while keeping the registered composition in the outer phase. Furthermore, process steps in tableting are reduced from eight (including sieving, granulating with internal and outer excipients, blending, compression, and cleaning) to just four (sieving, blending, compression, tableting).
Simplified formulations
Granulated Ibuprofen DC-100 has a granule size in the range between approximately 200 μm and 500 μm with a bulk density at 0.5 g/ml to 0.7 g/ml. It satisfies regulations following Ph. Eur. With a 100% API concentration the granulated Ibuprofen easily substitutes for conventional Ibuprofen in formulations that can provide either immediate or extended drug release profiles. Thus, Ibuprofen DC-100 can replace current Ibuprofen grade with only minor regulatory changes since no other excipient is employed. Ibuprofen DC-100 for direct compression is not only useful for tablets, but also for sachets or stick pack applications.
The high drug concentration also provides maximum freedom in formulation to optimize dosage forms (e.g., tablet size, release characteristics, and taste-masking).
Click on Ibuprofen DC100 grade for Direct Compression for further information
Click on Pharmatrans Ibuprofen DC 100 for product information
See the product information as PDF Flyer here:
(click to open PDF)

Contact:
Pharmatrans SANAQ AG
Gewerbestrasse 18
4123 Allschwil
Switzerland
Email: [email protected]
Tel. +41 61 225 90 00

