A study on the influence of the dissolution test factors on in vitro release of ibuprofen from sustained release tablets
Objectives
The aim of this study was to investigate the influence of in vitro release test parameters on the release of ibuprofen from sustained release inert matrix tablets.
Materials and methods
Ibuprofen sustained release inert matrix tablets were manufactured at a laboratory scale using Kollidon® SR as matrix formator. The variables of in vitro release study were: dissolution media pH (7.2-6.8-5.4-1.2), apparatus type (rotative basket and rotative paddle) and stirring speed (50 rpm and 100 rpm).
Outcomes.
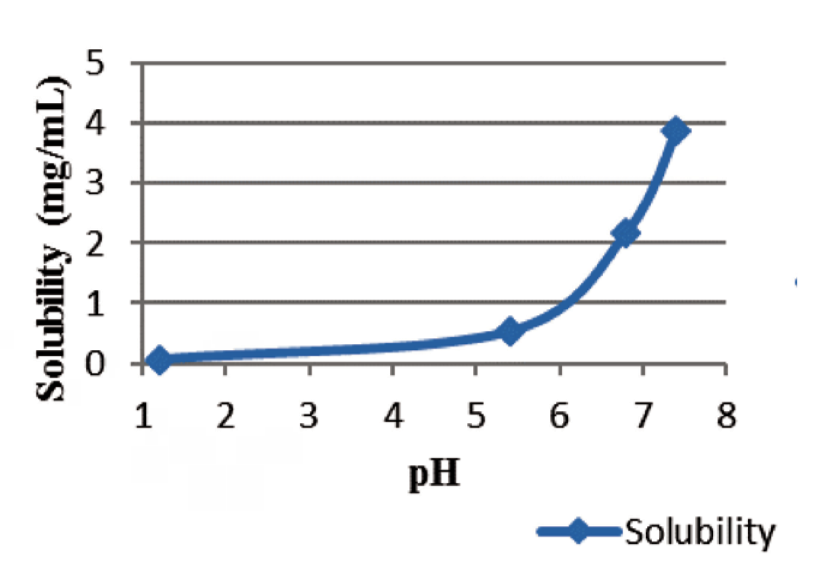
An increase in ibuprofen solubility together with an increase on pH value was observed. Percentage drug release increases together with the increase of pH. Rotative paddle apparatus proved to generate much powerful hydrody- namic forces in comparison with rotative basket apparatus which leads to a rapid in vitro release. Increasing stirring speed will determine a faster release of ibuprofen from matrix tablets. Statistical comparison of release profiles was per- formed by means of similarity factor f2. Both statistical release profiles comparison (between apparatuses and between stirring speeds) showed no influences of apparatus type and stirring speed on in vitro release at pH 1.2 and 5.4. At pH 6.8 and 7.2 there are strong influences of both apparatus type and stirring speed on ibuprofen release from matrix tablets. Modeling of release kinetics showed a good fitting by Weitbull and Korshmeyer-Peppas equations (especially at pH 6.8 and 7.2) and a fair fitting by Higuchi equation (at pH 1.2 and 5.4).
Conclusions
In vitro release of ibuprofen from sustained release matrix tablets depends on dissolution media pH and release rate depends on judicious choice of dissolution test factors to made precise in vitro/in vivo correlations.
Download the full study here: A study on the influence of the dissolution test factors on in vitro release of ibuprofen from sustained release tablets or continue reading here: Ref: Ro J Pharm Pract. 2020;13(2) DOI: 10.37897/RJPhP.2020.2.6 – https://FARMA.com.ro | PHARMACEUTICALPRACTICE – Corresponding author: Luca Liviu Rus
Keywords: Ibuprofen, Supertab 14 SD, Kollidon® SR, Aerosil 200, Magnesium stearate

