Elucidation of processing parameters for the reverse engineering of tablets
Abstract
Reverse engineering can assist in decoding the formula and manufacturing parameters employed in innovator formulations. Generic pharmaceutical industries use it to develop generic cheaper versions of innovator tablets. Herein, we report the systematic application of reverse engineering in determining the manufacturing process utilized by innovators to prepare tablet formulations. The outcome inferred that the critical information such as the granulation and solvent type in the innovator formulation could be identified by systematic analysis via scanning electron microscopy (SEM) images and sieve and texture analysis. Furthermore, critical investigation of the levels of fines generated during sieve analysis could reveal the tablet manufacturing process. It was observed that the maximum amount of fines was generated in the case of post-compression granules obtained by tablets prepared by direct compression. The hardness of granules is yet another major factor that could help to delineate the type of drying technique used in innovator manufacturing. Granules obtained from crushing a tablet prepared by wet granulation with tray drying were harder than those prepared by drying on a fluidized bed dryer (FBD). The outcome of this investigation may be helpful for formulation scientists working on the development of generic formulations.
Introduction
Several dosage forms are available for the treatment of allied diseases and disorders, including tablets, capsules, creams, ointments, suspensions, emulsions, gels, mixtures, pellets, lozenges, liniments, lotions, pastes, suppositories, sprays, and inhalants.1 Among these dosage forms, tablets are the most widely employed due to the advantages of a superior stability profile, high patient compliance, economical dosage form, ease of handling, ease of administration, and ease of transformation into specialized drug products (such as for delayed enteric release).2 The tablets prepared by innovators are usually costly due to the involvement of enormous research and development costs. Hence, the pharma industry is inclined to manufacture generic versions of innovator products.3 Mapping a generic version of a tablet with that of the innovator is a very tricky juncture in the development of generics.
Reverse engineering is defined as the process involving the identification, separation, and quantitation of individual components or ingredients in a drug product. The information concerning these process parameters is necessary to create a generic version of medication and requires various chemical processes to discover the exact contents of a formula.4 To achieve this goal, reverse engineering usually follows three steps, i.e., decoding the quantitative formula, solid-state characterization of the active pharmaceutical ingredient (API), and identifying the manufacturing process.
Once the formula for the dosage form is obtained, various separation processes have to be performed to separate all the ingredients for decoding the quantitative formula. Generally, the separation techniques used to separate the components include differential solubility, high-performance liquid chromatography (HPLC), and thin-layer chromatography.5
After separating all the ingredients, their quantitative analysis is carried out using different techniques such as gravimetric analysis, differential scanning calorimetry (DSC) and UV-visible spectroscopy.5 After the successful determination of the quantitative formula, in the second step, the characterization of the API is performed. In this step, many techniques are used to characterize the API, including DSC, which is used to identify the physical interaction between the API and the formulation excipients;6 Fourier-transform infrared spectroscopy (FTIR), which is used to find out any chemical interaction between the API and the excipients present;7 powder X-ray diffraction (XRD), which is used to check the polymorphic forms of the API;8 and microscopic techniques, which are executed to observe the morphology as well as to measure the particle size of the API.9
In the last step, the identification of the manufacturing step is carried out. In this step, the method used for manufacturing the tablets can be predicted based on the physicochemical properties of the API by checking the disintegration pattern under a microscope or by visual examination of tablet fracture.5
If the API is sensitive to moisture, then a wet granulation process cannot be employed to prepare the tablet, and so the tablets are prepared using direct compression (DC) or dry granulation method. If the API remains stable in moisture, it can be formulated into a tablet using any of three main processes. Hence, it becomes challenging to rely on one method to determine the process used for manufacturing.5,10
Further, the disintegration pattern of the tablet can be observed under a microscope by putting a small piece of tablet in a disintegrating medium under a microscope. During disintegration, if the tablet first disintegrates into granules and then into smaller particles, it can be inferred that the granulation technique was used to manufacture the tablet. If small particles are observed directly, then it can be concluded that the method used for manufacturing the tablet was DC. However, in this technique, it is also extremely difficult to identify the granulation method used to prepare the tablets, i.e., dry granulation or wet granulation.5
Fracture of the tablet can also be employed to confirm the manufacturing process of the tablet. If the fracture is smooth, then it infers that the DC method was used for manufacturing. On the other hand, if the fracture is rough, then granulation was used. It may be noted that the exact granulation process, i.e., whether wet or dry granulation has been used, cannot be precisely predicted.5 Hence, there is an urgent need to devise a rapid tool to identify the information concerning these process parameters toward creating a generic tablet formulation.
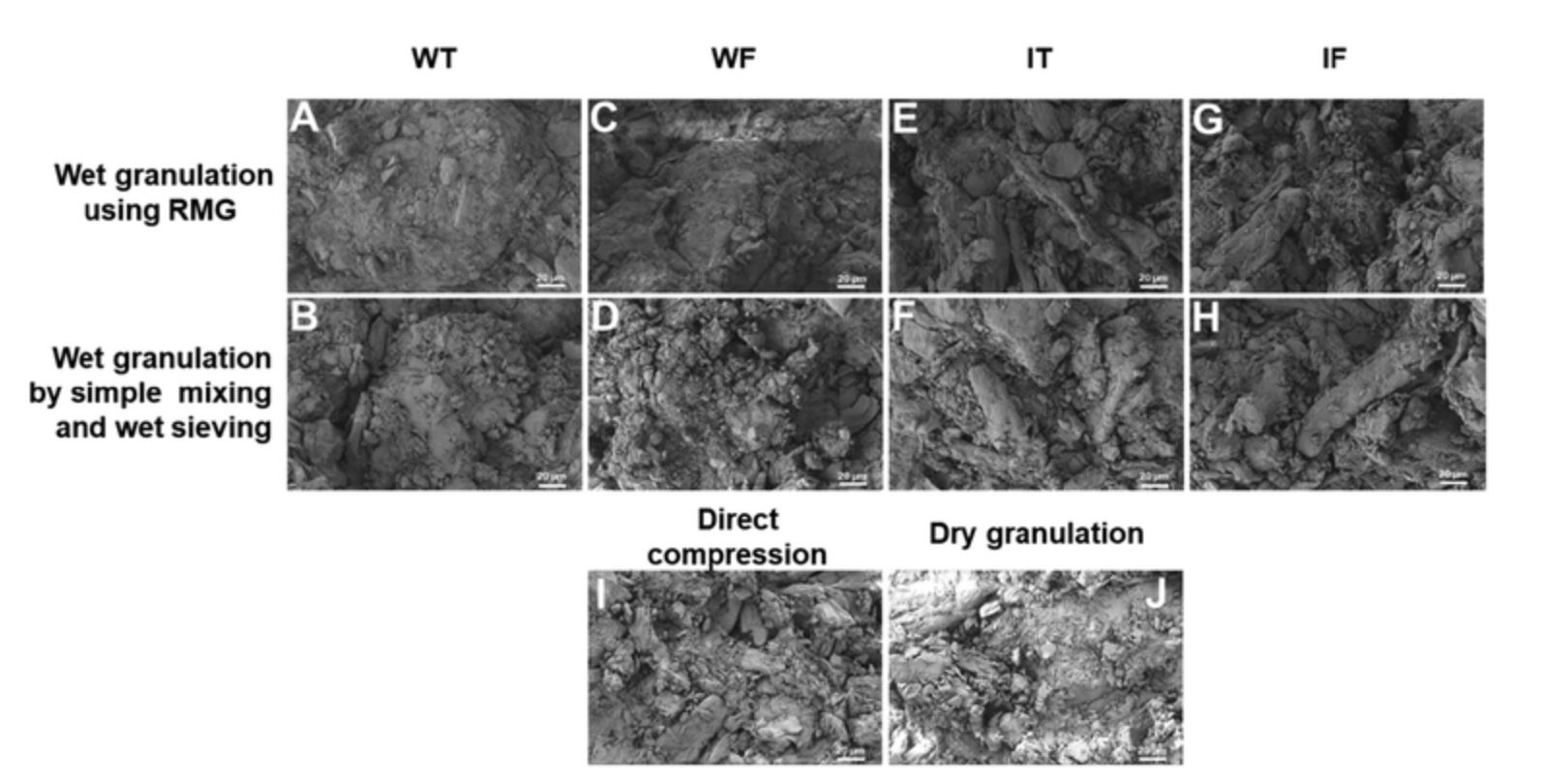
The tablet, being the most commonly exercised conventional dosage form, has been the most explored in research setups, leaving us with very narrow areas to explore in this domain. Researchers are already trying to fill knowledge gaps, enhance tablet quality, and accelerate the production of generic pharmaceuticals through methodical examinations of the various formulation elements, production procedures, and analytical techniques. Toward this avenue, this research reports different techniques to identify the characteristic features to conclude on the manufacturing process. Tablets prepared using different approaches were evaluated using various methods, including SEM, a texture analyzer, and a sieve shaker to determine the characteristic patterns associated with particular manufacturing processes. Hence, the reported literature is bound to possess overlapping similarities in respective studies. However, to the best of our knowledge we are the first to evaluate the effect of the method of granulation and the solvent used on the morphological features using SEM. Pre-compression and post-compression hardness studies are also distinctively featured in this manuscript.
Download the full article as PDF here Elucidation of processing parameters for the reverse engineering of tablets
or read it here
Materials
Lamivudine was received as a gift sample (Aurigene Discovery Technologies Limited, Bangalore, India). Lactose anhydrous (DuraLac® H) was purchased from Meggle Excipient and Technology (Mumbai, India). Microcrystalline cellulose (MCC, Avicel PH-101) and polyvinyl pyrrolidine (PVP) k-30 were purchased from FMC Biopolymer (Mumbai, India). Magnesium stearate was procured from Loba Chemie (Mumbai, India). Talc was procured from Luzenac Pharma (Mumbai, India), and isopropyl alcohol was purchased from Thermo Fischer Scientific Pvt. Ltd (Mumbai, India).
Devendra Choudhary, Dnyaneshwar Kalyane, Suryanarayana Polaka, Tanisha Gupta and Rakesh Kumar Tekade, Elucidation of processing parameters for the reverse engineering of tablets, Received 1st December 2023 , Accepted 3rd April 2024, First published on 22nd April 2024, DOI: 10.1039/D3PM00058C (Paper) RSC Pharm., 2024, Advance Article
Read also our introduction article on Talc here:


