Olopatadine hydrochloride loaded Kollidon® SR nanoparticles for ocular delivery: Nanosuspension formulation and in vitro–in vivo evaluation
Introduction
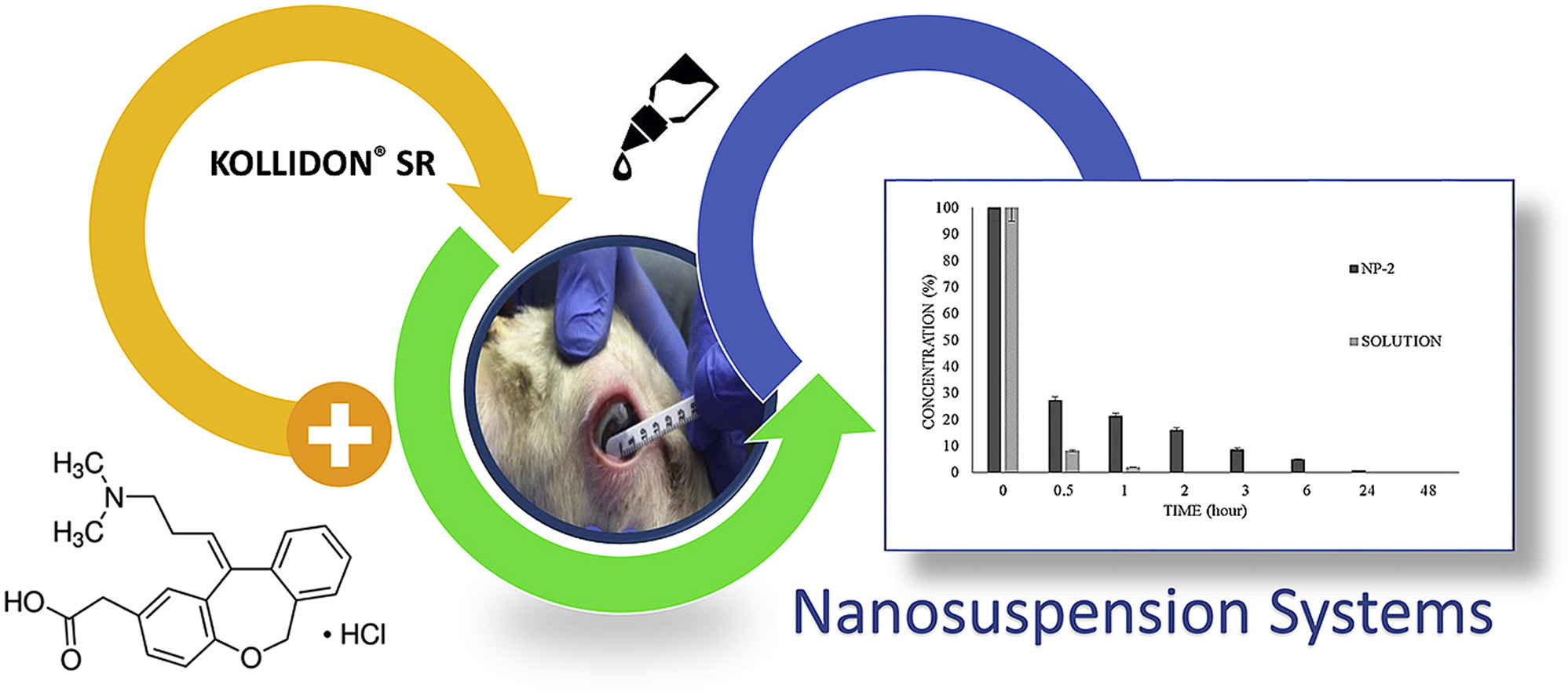
Allergic eye diseases includes a spectrum of diseases, with each state being characterized by a complicated immunopathology. In the present study, polymeric Kollidon® SR nanoparticles loaded with olopatadine hydrochloride (OLO) were formulated as a suspension for ocular drug delivery. The formulations were developed to treat ocular allergic disease and characterized by their improved duration of corneal retention.
Methods
OLO-loaded Kollidon® SR nanoparticles were prepared using the spray-drying method. The active agent was quantified using ultra-high performance liquid chromatography (U-HPLC). The nanoparticles were characterized according to entrapment efficiency, particle size, zeta potential, morphology, solid-state characterizations, and drug release. The in vitro release studies on OLO-loaded nanoparticles were examined in simulated tear fluid (pH 7.4). In vivo studies were conducted on healthy female/male Anatolian Merino sheep to investigate the retention time of the active agent.
Results and Discussion
Characterization studies showed that OLO was successfully loaded into the nanoparticle system and that the suspension formulation is suitable for ocular administration. Using DDSolver, the in vitro release of the OLO from nanoparticle formulations followed the Korsmeyer-Peppas model.
Conclusions
The proposed spray-drying method can be successfully applied for preparing OLO-loaded nanoparticles without adding crosslinkers. OLO-loaded nanoparticles appear to be a promising extended-release drug delivery system that can be used to treat ocular diseases with a more prolonged drug-residence time on the ocular surface.
Article Information: Umay Merve Güven, Evrim Yenilmez; Sciencedirect, 2019

