Taste-Masked Pellets of Warfarin Sodium: Formulation towards the Dose Personalisation
Abstract
Introduction
The main disadvantage of most oral mass-market drug products is that they do not fit everyone. The reason usually lies in the required personalised approach, including dose personalisation or consumer properties [1,2]. Inappropriate consumer properties are usually associated with unpleasant smells, tastes, swallowing discomfort, or even swallowing problems (such as dysphagia) [3]. Some paediatric age subgroups have contraindications to be treated with tablets and capsules at the same time and, for the geriatric population subgroup, swallowing discomfort is acquired with age because of decreased motor activities [4,5]. It is worth mentioning that swallowing discomfort is observed often, even in healthy adults [6].
The usual real-world approach to solving the swallowing problem is to break or crush the tablet or open the capsule [7]. The problem with this is that the biopharmaceutical properties of drug products can change drastically due to changes in the dissolution profile and the place of drug release [8,9]. In addition, after these unauthorised modifications, the unpleasant smell and taste problems intensify.
One of the drugs that requires both dose personalisation and taste masking [10] is warfarin [11]. Warfarin is a coumarin anticoagulant and a vitamin K antagonist used to treat and originally prevent thromboembolic events and the development of the disease in cases of many different health conditions, such as deep venous thrombosis, cardiomyopathy, and pulmonary embolism. It is also used for thrombosis, stroke, and myocardial infarction prophylaxis, among others. Warfarin inhibits the vitamin K-dependent synthesis of coagulation factors (II, VII, IX, and X) by inhibiting vitamin K reductase, vitamin K epoxide reductase, and anticoagulant proteins C and S, therefore depleting the available vitamin K-dependent activated proteins that play a role in the coagulation process. At the beginning of the treatment, a certain loading dose is needed. Due to the mechanism of action, the first effect is seen gradually, and the full clinical effect can be observed 3–4 days after starting the therapy. Based on the indication and considering its narrow therapeutic index, the general oral dose may vary from 2 to 10 mg once a day, depending on age, weight, genetic polymorphism, and the prothrombin time [12].
Thus, the personalisation of the warfarin dose is demanded. Patients may greatly benefit from changes in dosage using different drug dosage forms as well as oral dosage forms that can still be taste-masked against the bitter taste of warfarin, while keeping the flexibility of personalised dosing [10,11,13].
One of the approaches to solving taste and swallowing problems is to formulate the drug in the form of a liquid suspension or solution. Taste masking is usually achieved by adding taste additives and sweeteners [10]. Despite the relative effectiveness of this method, solutions and suspensions cannot solve the taste-masking problem completely because they cannot prevent the drugs from come into contact with the taste buds [10,14,15].
To the best of our knowledge, the best alternative approach is represented by taste-masked microparticles [14,16,17]. This approach provides better taste masking due to the microparticles, which are insoluble in oral cavity saliva with a pH of 5.8–7.6 [18]. Thus, the drug does not come into contact with the taste buds. The insolubility of particles in oral liquids can be achieved by using polymers with pH-dependent solubility, such as Kollicoat® Smartseal 30D. This polymer is insoluble at the pH of the oral cavity, but dissolves quickly at the pH of the stomach [16,19].
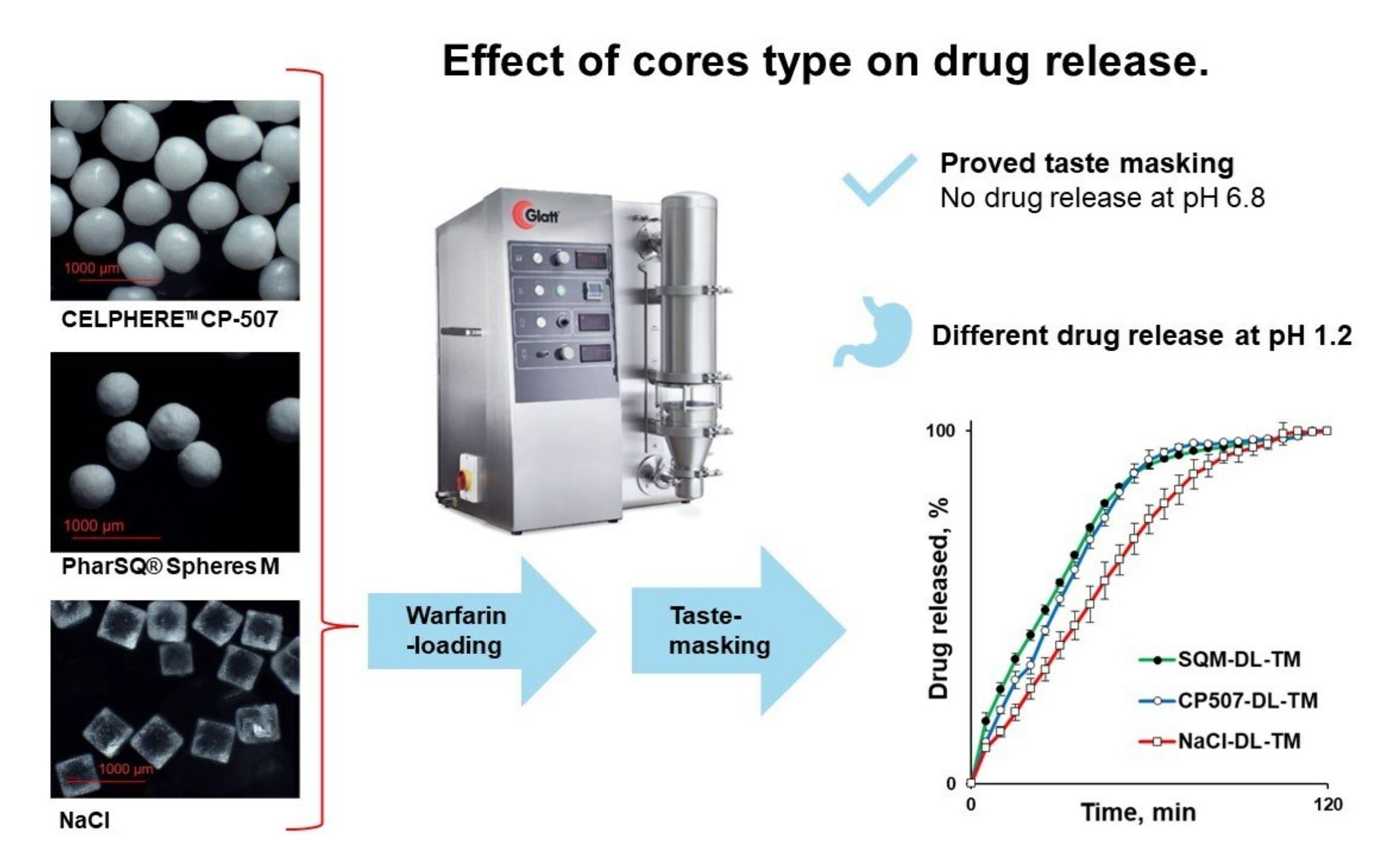
The taste-masked microparticles/pellets can be manufactured by coating drug-containing granules or by drug-layering placebo cores and then applying a taste-masking coating [20,21]. Even though placebo cores are usually perceived as inert, the cores’ properties can affect the drug release from the pellets. For instance, the density of the particles predetermines the number of particles per gram, the size of the cores predetermines the specific surface area, and the solubility and osmolarity can increase the release rate [22,23,24].
While the application of Kollicoat® Smartseal to obtain taste-masking-coated pellets is known, to the best of our knowledge, this polymer has never been investigated before for the preparation of warfarin-containing pellets. Moreover, the effect of the core type on the release of warfarin from the Kollicoat® Smartseal-coated pellets has not yet been investigated.
The aim of this study is to investigate the effect of microcrystalline cellulose, anhydrous dibasic calcium phosphate, and sodium chloride cores of comparable sizes in terms of their effect on the release of warfarin from the Kollicoat® Smartseal taste-masking-coated pellets.
Download the full article as PDF here: Taste-Masked Pellets of Warfarin Sodium
or read it here
Materials
Warfarin sodium clathrate (batch # WA22003; Alchymars Icm SM Private Ltd., Tamil, Nadu, India) was used as an active pharmaceutical ingredient. Microcrystalline cellulose (MCC) spheroids (CELPHERE™ CP-507 (500–710 µm); Asahi Kasei Co., Tokyo, Japan), dibasic calcium phosphate anhydrous (80 wt.%) and microcrystalline cellulose (20 wt.%) spheroids (PharSQ® Spheres M (500–710 µm); Budenheim KG, Budenheim, Germany) [25,26], and sodium chloride (NaCl; Valdo SIA, Riga, Latvia) sieved particles (500–800 µm fraction) were used as cores of pellets. Hydroxypropyl methylcellulose (HPMC; Methocel™ E5 premium LV; Dow Chemical, Midland, MI, USA) was used as a binder for drug loading (Figure 1A). Co-polymer comprising methyl methacrylate (MMA) and diethylaminoehtylmethacrylate (DEAEMA) in the ratio 7:3 in the form of aqueous dispersion Kollicoat® Smartseal 30D (BASF SE, Ludwigshafen, Germany) was used as a taste-masking coating material (Figure 1B) [27] and dibutyl sebacate (DBS; Merck KGaA, Darmstadt, Germany) as a plasticiser [28]. A synthetic colloidal silicon dioxide (Syloid® 244FP; Grace GmbH, Worms, Germany) was used as anti-tacking/-caking agent. Hydrochloric acid (37%), potassium dihydrogen phosphate, and disodium hydrogen phosphate (analytical grade) were supplied by Merck KGaA (Darmstadt, Germany).
Drug Layering
The HPMC containing [13] aqueous-based warfarin sodium clathrate layering solution [30] with a solid content of 30 wt.% (Table 1) was sprayed onto cores in a fluidised bed coater ( Mini-Glatt, Glatt GmbH, Binzen, Germany) with a Wurster tube (positioned at 12 mm above the bottom of the processing chamber) to achieve 30 wt.% weight gain (28.7 wt.% weight gain due to the warfarin sodium) per 100 g of cores.
Kovalenko, L.; Kukuls, K.; Berga, M.; Mohylyuk, V. Taste-Masked Pellets of Warfarin Sodium: Formulation towards the Dose Personalisation. Pharmaceutics 2024, 16, 586. https://doi.org/10.3390/pharmaceutics16050586

