Preparation and evaluation of β-cyclodextrin-based nanosponges loaded with Budesonide for pulmonary delivery
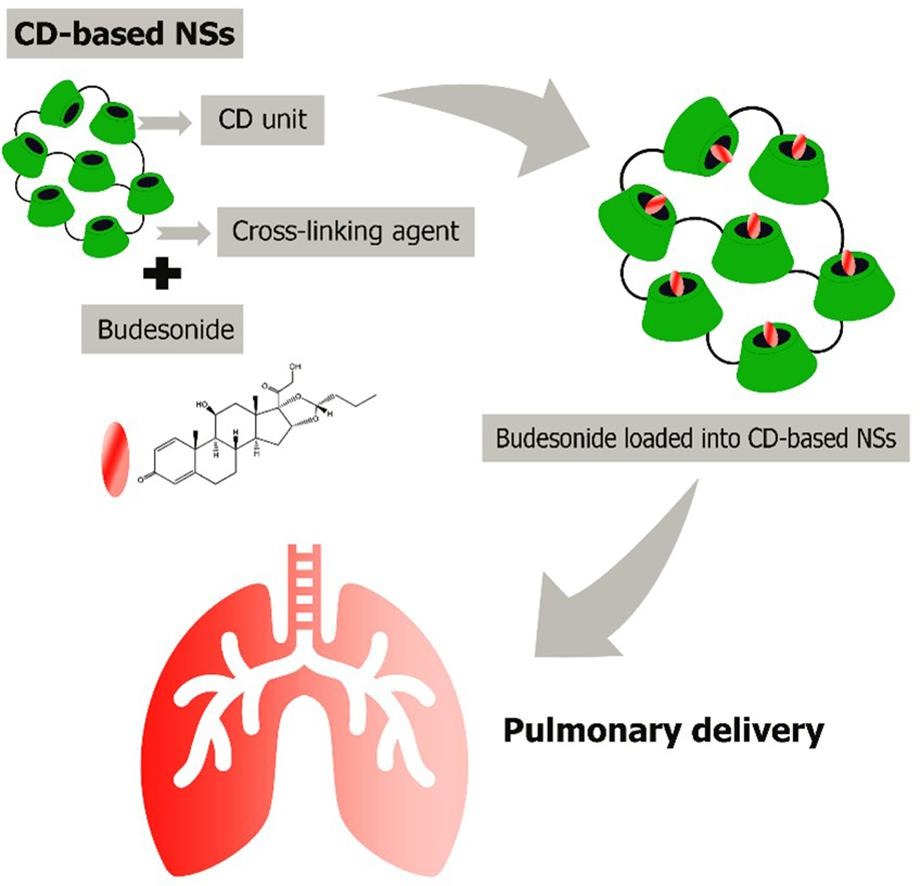
Budesonide (BUD) is a glucocorticosteroid used to treat chronic obstructive pulmonary disease. Despite this, it is a hydrophobic compound with low bioavailability. To address these hurdles, non-toxic and biocompatible βcyclodextrin-based nanosponges (βCD-NS) were attempted. BUD was loaded on five different βCD-NS at four different ratios. NS with 1,1′-carbonyldiimidazole (CDI) as a crosslinking agent, presented a higher encapsulation efficiency ( ̴ 80%) of BUD at 1:3 BUD: βCD-NS ratio (BUD-βCD-NS). The optimized formulations were characterized by Fourier-transform infrared spectroscopy (FTIR), thermogravimetric analysis (TGA), water absorption capacity (WAC), scanning electron microscopy (SEM), X-ray powder diffraction studies (XRD), particle size, zeta potential, encapsulation efficiency, in vitro and in vivo release studies, acute toxicity study, solid-state characterization, and aerosol performance. In vitro-in vivo correlation and cytotoxicity of the formulations on alveolar cells in vitro were further determined. In vitro and in vivo studies showed almost complete drug release and drug absorption from the lungs in the initial 2 h for pure BUD, which were sustained up to 12 h from BUD loaded into nanosponges (BUD-βCD-NS). Acute toxicity studies and in vitro cytotoxicity studies on alveolar cells proved the safety of BUD-βCD-NS. Several parameters, including particle size, median mass aerodynamic diameter, % fine particle fraction, and % emitted dose, were evaluated for aerosol performance, suggesting the capability of BUD-βCD-NS to formulate as a dry powder inhaler (DPI) with a suitable diluent. To sum up, this research will offer new insights into the future advancement of βCD-NS as drug delivery systems for providing controlled release of therapeutic agents against pulmonary disease.
Reagents
Dextrins such as β-Cyclodextrin (βCD; Mw = 1134.98 g/mol) and KLEPTOSE® Linecaps (LC; Mw = ∼12,000 g/mol), were kindly provided as a gift by Roquette (Lestrem, France). Dextrins were dried in the oven at 80 0C up to constant weight before their usage to remove any moisture content. Pyromellitic dianhydride (PMDA, 97 %); Dimethylsulfoxide (DMSO, (≥99.90 %); Triethylamine (Et3N, ≥99.00 %); Acetone (≥99.00 % (GC)); 1, 1′-Carbonyldiimidazole (CDI, ≥97.00 %); Sodium hypophosphite monohydrate (SHP,…
Read more
Yasmein Yaser Salem, Gjylije Hoti, Rana M.F. Sammour, Fabrizio Caldera, Claudio Cecone, Adrián Matencio, Aliasgar F. Shahiwala, Francesco Trotta, Preparation and evaluation of βcyclodextrin-based nanosponges loaded with Budesonide for pulmonary delivery, International Journal of Pharmaceutics, Volume 647, 2023, 123529, ISSN 0378-5173, https://doi.org/10.1016/j.ijpharm.2023.123529.
See the webinar:
“Rational Selection of Cyclodextrins for the Solubilization of Poorly Soluble Oral Drugs”, 8. November 2023:
Get more information & register here for free:


