3D-Printed Lipid Mesophases for the Treatment of Chronic Liver Disease
Abstract
Although lipid-based formulations are an attractive approach for enhancing the oral bioavailability of lipophilic drugs, their addition into solid oral dosage forms has been proven challenging due to their high viscosity and heat sensitivity. Therefore, unlike the traditional tableting process, this study employed semi-solid extrusion 3D-printing to produce–at room temperature–gastro-resistant printlets containing a high percentage of bioactive lipids for the effective delivery of lipophilic drugs through self-emulsification. The bio-compatible lipidic mesophase ink, owing to a tunable 3D nanostructure, is employed as a starting material to produce printlets via additive manufacturing. An active lipid mixture – with antifibrotic properties – is blended with the antioxidant vitamin E and water, and the ink printability is optimized by carefully tailoring its composition, and thus its phase identity. The obtained printlets disintegrated upon contact with intestinal fluids forming colloidal structures that enhanced the solubility of a poorly water-soluble drug. The printlets exhibited antifibrotic activity on human hepatic stellate cells, LX-2, suggesting that the generated self-emulsified colloidal structures made both the fibrosis-resolving bioactive excipients and the drug promptly available, enhancing their cell uptake and, in turn, their therapeutic activity.
Introduction
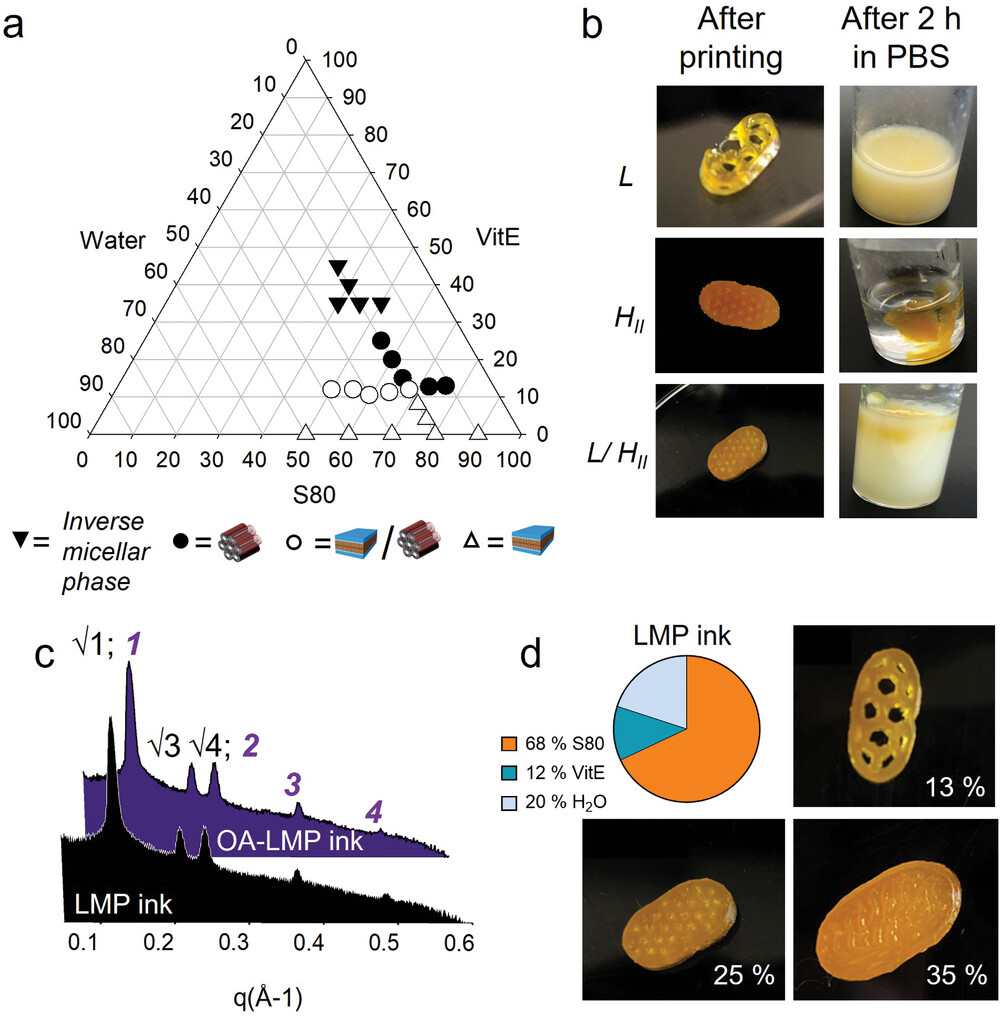
Lipid-based formulations offer an attractive approach for enhancing the oral bioavailability of several lipophilic drugs[1] thanks to the formation of colloidal particles within the gastrointestinal (GI) tract that ameliorate drug solubility.[2] Despite the commercial and clinical success of various delivery systems,[3] the addition of lipids in solid oral dosage forms has been proven challenging due to their limited versatility with respect to other traditional excipients used to produce tablets.[4] The application of 3D-printing in pharmaceutics and personalized medicine has garnered significant interest in the field.[5-8] Namely, 3D-printing technology enables customizable dosing,[9] manufacturing of polypills,[10-14] and offers tunable drug release.[15-19] This is exemplified by the approval of the first commercially available 3D-printed medication, Spritam, by the United States Food and Drug Administration (FDA) in 2015,[20] which also demonstrated the feasibility of using 3D-printing in large-scale manufacturing.[5, 21] Given the high viscosity and heat-sensitivity of lipids, and to facilitate broader drug applicability, our study employs semi-solid extrusion (SSE) 3D-printing technology to produce oral dosage forms containing a high percentage of bioactive lipids for the effective delivery of water-insoluble drugs through self-emulsification. Unlike the traditional tableting process, SSE does not rely on compression forces but instead utilizes a viscous matrix to hold layers of formulation together producing the dosage form at room temperature,[22, 23] which is of great importance for thermolabile lipids and active principles. Recently, 3D-printed solid lipid formulations have been described, but to our knowledge always as a blend of lipids and polymers to obviate the suboptimal mechanical properties of the lipids employed.[8, 4, 12] Here, we selected lipid mesophases (LMPs) as a structured biomaterial to be used as ink for additive manufacturing of 3D printed tablets (printlets).
LMPs are a class of materials belonging to the lyotropic-liquid-crystal family, possessing a tuneable three-dimensional nanostructure along with mechanical and rheological properties (e.g., flexural strength and rigidity) of high interest for efficient material engineering for 3D printing. By altering the water content and temperature or incorporating additives, we can tailor the lipid mesophase to exhibit lamellar (L), cubic (Q), and inverse hexagonal (HII), or micellar geometries.[24] Each phase geometry has an impact on drug release rates, viscosity, and self-emulsification mechanisms. By exploring the nanostructure-property correlations, lipid matrices with the desired material and self-emulsification properties were developed. While extensively investigated – either as bulk gel or as colloidal dispersion – to achieve controlled drug release,[25-28] LMP-based formulations have not been reported so far as starting material to manufacture pharmaceutical oral dosage forms via 3D printing technology. Here, to generate our LMP-based ink (LMP ink) we selected a natural phospholipid from soy, S80, rich in polyenylphosphatidylcholines (PPC) at a concentration greater than 75%. Among the possible candidates able to form LMPs such as phosphatidylcholine, monoacylglycerol lipids (monoolein, monolinolein) and phytantriol, S80 also works as an active ingredient able to deactivate profibrogenic hepatic stellate cells, which are the main collagen-producing cells in hepatic fibrogenesis.[29] Nonetheless, pure unsaturated lipids are unsuitable for 3D-printing due to their intrinsic waxy and soft nature. Therefore, we chose to blend S80 with vitamin E (VitE) because of its demonstrated capacity to promote the formation of a highly viscous gel[30-32] exhibiting an elastic behaviour with a response to shear[33, 34] as well as its well-known antioxidant properties that prevent lipid peroxidation.[35]
The poorly water-soluble (logP = 5.7) and highly permeable obeticholic acid (OA; classified as Class II according to the Biopharmaceutics Classification System) represents an ideal active principle to determine the utility of the developed formulation. OA is a semi-synthetic bile acid analogue, acting as agonist of the farnesoid X receptor,[36] and marketed as film-coated tablets approved as orphan medicine for the treatment of primary biliary cholangitis, an autoimmune condition in which there is a gradual destruction of the small bile ducts in the liver.[37] In preclinical studies, OA has also been shown to improve hepatic steatosis, fibrosis, and portal hypertension.[38-41] Despite the encouraging results from clinical studies of their OA tablet formulation Ocaliva against placebo, the company Intercept Pharmaceuticals has been recently denied accelerated approval for the treatment of patients with pre-cirrhotic liver fibrosis due to nonalcoholic steatohepatitis (NASH). Based on the outcome of the randomized global Phase 3 study REGENERATE, US Food and Drug Administration experts concluded that the benefits of Ocaliva do not outweigh the risks in NASH patients with stage 2 or 3 fibrosis. Our recent research on hepatic stellate cells (HSCs), key players in the fibrogenic process, suggests that formulating OA with PPC-based dosage forms could mitigate the possibly detrimental effect of the drug on HSCs and favor a healthy cell phenotype, thus re-igniting the potential of clinical success.[42]
Our approach has led to i) the design of an LMP ink for additive manufacturing containing a high content of bioactive lipid; ii) the incorporation of the water-insoluble OA into the printable LMP ink (OA-LMP ink) at a dose comparable to the marketed one; iii) the production of uniform 3D-printed tablets (printlets) using SSE at room temperature; iv) the disintegration of the printlets in intestinal fluids through self-emulsification and v) the increase in the solubility of the embedded drug in the selected intestinal fluids. Our printlets show potential for the treatment of chronic liver disease and they can serve as a versatile platform for the printing of other poorly water-soluble drugs for oral delivery.
Conclusion
In our study, we have successfully demonstrated the underexplored potential of lipid mesophases as a printable material suitable for semi-solid extrusion at room temperature. By carefully controlling the ink composition and thus the phase identity of the ensuing ink we can in turn fine tune its rheology and optimize the printability. We were thus able to produce printlets with defined infill densities and overall physical parameters. Our formulation is characterized by a high percentage of a natural phospholipid, generally regarded as safe (GRAS) by the US Food and Drug Administration, with antifibrotic properties, rendering it an exceptional candidate for the delivery of water-insoluble drugs. Upon contact with intestinal fluids, the printlets disintegrated forming colloidal structures that enhanced the solubility of the water-insoluble drug, OA. Remarkably, the printlets and the corresponding 3D structures of the gel are able to retain OA, enabling its slow and controlled release in simulated biorelevant intestinal fluids. Our results suggest that the LMP-based printlets have potential as oral treatment, since the self-emulsifying system generated upon contact with intestinal fluids does not affect either the intestinal cell viability or their permeability. Thanks to the increase in solubility of OA and the co-formulation with known hepatoprotectants such as the PPC-rich S80 and VitE, LMP-based printlets offer a modular playground for the pharmacological treatment of liver fibrosis and can serve as a platform for 3D-printing other poorly water-soluble drugs. The use of LMPs in additive manufacturing paves new avenues not only for multifunctional oral dosage forms but also for bioderived scaffolds based on this material. However, further in vivo studies are needed to test the efficacy of 3D printed formulations using LMP inks. Although the feasibility of using 3D-printing in large-scale manufacturing has been already proven for oral dosage forms such as immediate release tablets, a scale-up study for semi-solid extrusion of purely lipid-based inks is needed to investigate possible bottlenecks in large scale production of LMP printlets.
Download the full article as PDF here: 3D-Printed Lipid Mesophases for the Treatment of Chronic Liver Disease
or read it here
Materials
S80 with 75% polyenylphosphatidylcholines was a kind gift from Lipoid GmbH (Ludwigshafen, Germany). The specific composition of this lipid mixture was reported in the Supporting Information (Table S5, Supporting Information). VitE (Ph. Eur. Quality) and 3-(4,5-dimethylthiazol-2-yl)−2,5-diphenyltetrazolium bromide (MTT) and porcine pancreatin (8 X USP specifications activity) were purchased from Sigma-Aldrich (St. Louis, USA). OA was purchased from abcr GmbH (Karlsruhe, Germany). Simulated fluids were purchased from Biorelevant (London, UK). Phosphate buffer saline and non-essential amino acids (NEAA) were purchased from Carl Roth (Karlsruhe, Germany). All organic solvents (methanol, acetonitrile, tetrahydrofuran (THF), dimethyl sulfoxide (DMSO) and Lucifer Yellow lithium salt were obtained from Fisher Scientific (Schwerte, Germany). Dulbecco’s Modification of Eagle’s Medium (DMEM), 1X with 4.5 g L−1 glucose, L-glutamine and sodium pyruvate was purchased from Costar Corning (Corning, USA). Penicillin/Streptomycin solution (100X) was purchased from Carlo Erba, L-Glutamine 100X (200 mM) was purchased from Microgem, Fetal Bovine Serum (FBS) and trypsin-EDTA 1X in PBS w/o Phenol Red, w/o Calcium, w/o Magnesium Sterile Filtered were purchased from EuroClone (Milan, Italy). Transwell cellQART 12-well Cell Culture Inserts, 0.4 µm PET clear were purchased from SABEU GmbH (Northeim, Germany). All chemicals were used as received. Ultrapure water of resistivity 18.2 MΩ.cm was produced by a Barnstead Smart2 pure device from Thermo Scientific (Pittsburgh, USA).
Marianna Carone, Rafaela Gazzi, Remo Eugster, Rita Gelli, Niklaas Manten, Aymar A. Ganguin, Silvia Di Valerio, Garima Yadav, Pasqualina Castaldo, Raffaele Mezzenga, Paola Luciani, Simone Aleandri, 3D-Printed Lipid Mesophases for the Treatment of Chronic Liver Disease, DOI: 10.1002/admt.202301930
Read also our introduction article on 3D Printing here:


