Using AI/ML to predict blending performance and process sensitivity for Continuous Direct Compression (CDC)
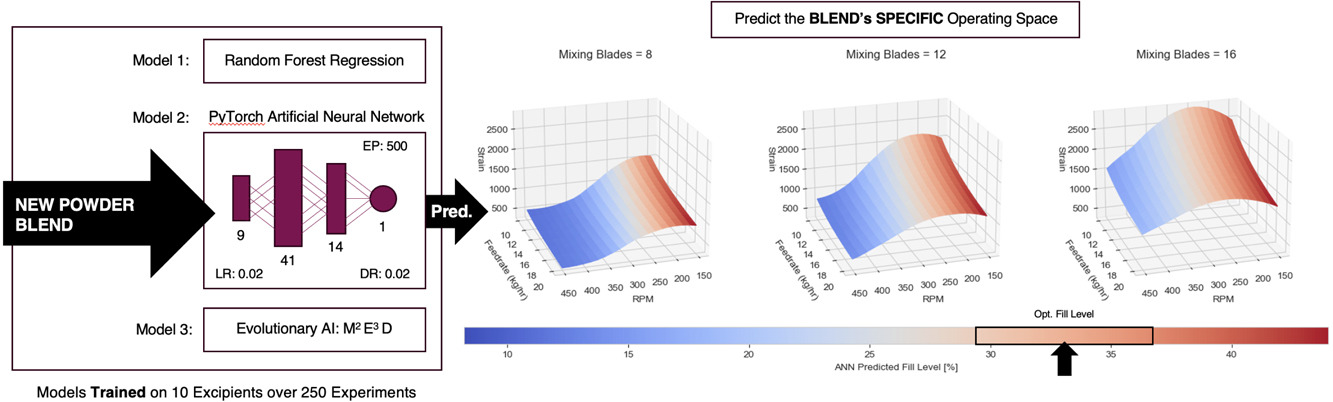
Utilising three artificial intelligence (AI)/machine learning (ML) tools, this study explores the prediction of fill level in inclined linear blenders at steady state by mapping a wide range of bulk powder characteristics to processing parameters. Predicting fill levels enables the calculation of blade passes (strain), known from existing literature to enhance content uniformity. We present and train three AI/ML models, each demonstrating unique predictive capabilities for fill level. These models collectively identify the following rank order of feature importance: RPM, Mixing Blade Region (MB) size, Wall Friction Angle (WFA), and Feed Rate (FR).
Random Forest Regression, a machine learning algorithm that constructs a multitude of decision trees at training time and outputs the mode of the classes (classification) or mean prediction (regression) of the individual trees, develops a series of individually useful decision trees. but also allows the extraction of logic and breakpoints within the data. A novel tool which utilises smart optimisation and symbolic regression to model complex systems into simple, closed-form equations, is used to build an accurate reduced-order model. Finally, an Artificial Neural Network (ANN), though less interrogable emerges as the most accurate fill level predictor, with an r² value of 0.97. Following training on single-component mixtures, the models are tested with a four-component powdered paracetamol formulation, mimicking an existing commercial drug product.
The ANN predicts the fill level of this formulation at three RPMs (250, 350 and 450) with a mean absolute error of 1.4%. Ultimately, the modelling tools showcase a framework to better understand the interaction between process and formulation. The result of this allows for a first-time-right approach for formulation development whilst gaining process understanding from fewer experiments. Resulting in the ability to approach risk during product development whilst gaining a greater holistic understanding of the processing environment of the desired formulation.
Download the full article as PDF here Using AI/ML to predict blending performance and process sensitivity for Continuous Direct Compression (CDC)
or read it here
Materials
Nine commonly used commercial excipients were used in this study, comprising: Avicel PH-101, Microcrystalline Cellulose, abbreviated to ‘PH-101’ (DuPont, Cork, Ireland), Avicel PH-102, Microcrystalline Cellulose, abbreviated to ‘PH-102’ (DuPont, Cork, Ireland), Avicel PH-105, Microcrystalline Cellulose, abbreviated to ‘PH-105’ (DuPont, Cork, Ireland), Vivapur 350, Microcrystalline Cellulose, abbreviated to ‘VP350’ (JRS Pharma, Rosenberg, Germany), Fast Flo 316, Lactose Monohydrate, abbreviated to ‘FF316’ (Kerry, Tralee, Ireland), Pharmatose 110M, Lactose Monohydrate, abbreviated to ‘PH110M’ (DFE Pharma, Veghel, Holland), Pharmatose 200M, Lactose Monohydrate, abbreviated to ‘PH200M’ (DFE Pharma, Veghel, Holland), Pharmatose 450M, Lactose Monohydrate, abbreviated to ‘PH450M’ (DFE Pharma, Veghel, Holland), A-Tab, Dicalcium Phosphate Anhydrous, abbreviated to ’A-Tab’ (Innophos, New Jersey, USA), and Pearlitol 200SD, Mannitol, abbreviated to ‘PT200SD’ (Roquette, Lestrem, France). In addition, the formulation utilises two additional materials: Powdered Paracetamol, abbreviated to ‘pAPAP’ (Mallinckrodt, USA) and Gycolys, Sodium starch glycolate (SSG), abbreviated to ‘Gycolys’ (Roquette, Lestrem, France). Furthermore, the drug surrogate formulation (F) in Table 1, is made up of 4 materials at the following weight percentages: Powdered Paracetamol (27% w/w), Avicel PH-102 (41% w/w), DCPA A-TAB (27% w/w), and Glycolys (5% w/w).
O. Jones-Salkey, C.R.K. Windows-Yule, A. Ingram, L. Stahler, A.L. Nicusan, S. Clifford, L. Martin de Juan, G.K. Reynolds, Using AI/ML to predict blending performance and process sensitivity for Continuous Direct Compression (CDC), International Journal of Pharmaceutics, Volume 651, 2024, 123796, ISSN 0378-5173, https://doi.org/10.1016/j.ijpharm.2024.123796.
Read more on “Orally Disintegrating Tablets (ODTs)” here:


