Development of inhalation powders containing lactic acid bacteria with antimicrobial activity against Pseudomonas aeruginosa
Abstract
Objectives
The aim of the project was to develop and characterise powders containing a probiotic (Lactiplantibacillus plantarum [Lpb. plantarum], Lacticaseibacillus rhamnosus, or Lactobacillus acidophilus) to be administered to the lung for the containment of pathogen growth in patients with lung infections.
Methods
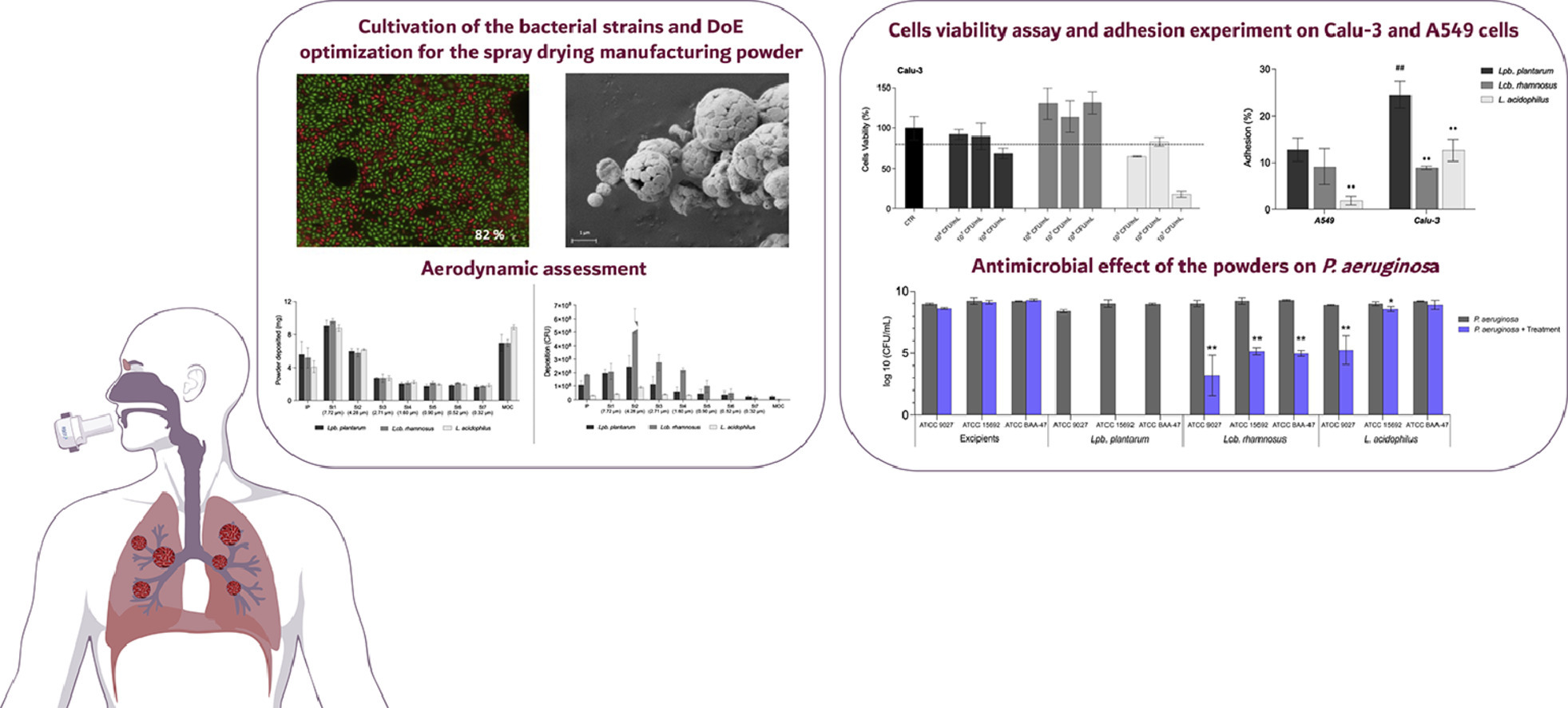
The optimised spray drying process for the powder manufacturing was able to preserve viability of the bacteria, which decreased of only one log unit and was maintained up to 30 days.
Highlights
- Acid lactic bacteria inhalation powders for lung infection.
- Optimized spray-drying production preserving lactobacilli viability.
- High in vitro respirability suitable for an efficient lung deposition.
- Adhesion capacity on A549 and Calu-3 pulmonary cell lines.
- Lpb. plantarum inhalation powder had bactericidal activity against P. aeruginosa.
Probiotic powders showed a high respirability (42%–50% of particles had a size < 5 µm) suitable for lung deposition and were proven safe on A549 and Calu-3 cells up to a concentration of 107 colony-forming units/mL. The Lpb. plantarum adhesion to both cell lines tested was at least 10%. Surprisingly, Lpb. plantarum powder was bactericidal at a concentration of 106 colony-forming units/mL on P. aeruginosa, whereas the other two strains were bacteriostatic.
Conclusion
This work represents a promising starting point to consider a probiotic inhalation powder a value in keeping the growth of pathogenic microflora in check during the antibiotic inhalation therapy suspension in cystic fibrosis treatment regimen. This approach could also be advantageous for interfering competitively with pathogenic bacteria and promoting the restoration of the healthy microbiota.
Download the full article as PDF here Development of inhalation powders containing lactic acid bacteria with antimicrobial activity against Pseudomonas aeruginosa
or read it here
Preliminary investigation and optimization of probiotic powders
Spray-dried powders were produced using a B-290 Mini Spray Dryer (Buchi, Switzerland) starting from 50 mL of Lpb. plantarum suspension in PBS or Ringer’s solution (VWR) at three different cell concentrations: 109, 5 × 109, and 1010 colony-forming units (CFU)/mL. The parameters set were: inlet temperature of 135°C, air flow rate at 600 L/h, aspiration at 35 m3/h, feed rate at 4 mL/min, and nozzle of 0.7 mm.
Then, the manufacturing process was optimised by applying a central composite Design of Experiments (DoE) with three factors and two levels (Table 1), and the significance of the model was calculated using the Design-Expert v13 software (Stat-Ease Inc., USA). The excipient consisted of lactose (Respitose ML001; DFE pharma, Germany) and L-leucine (ACEF Spa, Italy) in a ratio of 70:30 (w/w) and added in such quantities as to reach the concentration indicated by the DoE for each run.
Stefania Glieca, Eride Quarta, Benedetta Bottari, Elena Bancalari, Saverio Monica, Erika Scaltriti, Martina Tambassi, Lisa Flammini, Simona Bertoni, Annalisa Bianchera, Valentina Fainardi, Susanna Esposito, Giovanna Pisi, Ruggero Bettini, Fabio Sonvico, Francesca Buttini, Development of inhalation powders containing lactic acid bacteria with antimicrobial activity against Pseudomonas aeruginosa, International Journal of Antimicrobial Agents, Volume 63, Issue 1, 2024, 107001, ISSN 0924-8579, https://doi.org/10.1016/j.ijantimicag.2023.107001.
See also:
InhaLac® 145 – MEGGLE launches new milled lactose grade