Integrated Purification and Formulation of an Active Pharmaceutical Ingredient via Agitated Bed Crystallization and Fluidized Bed Processing
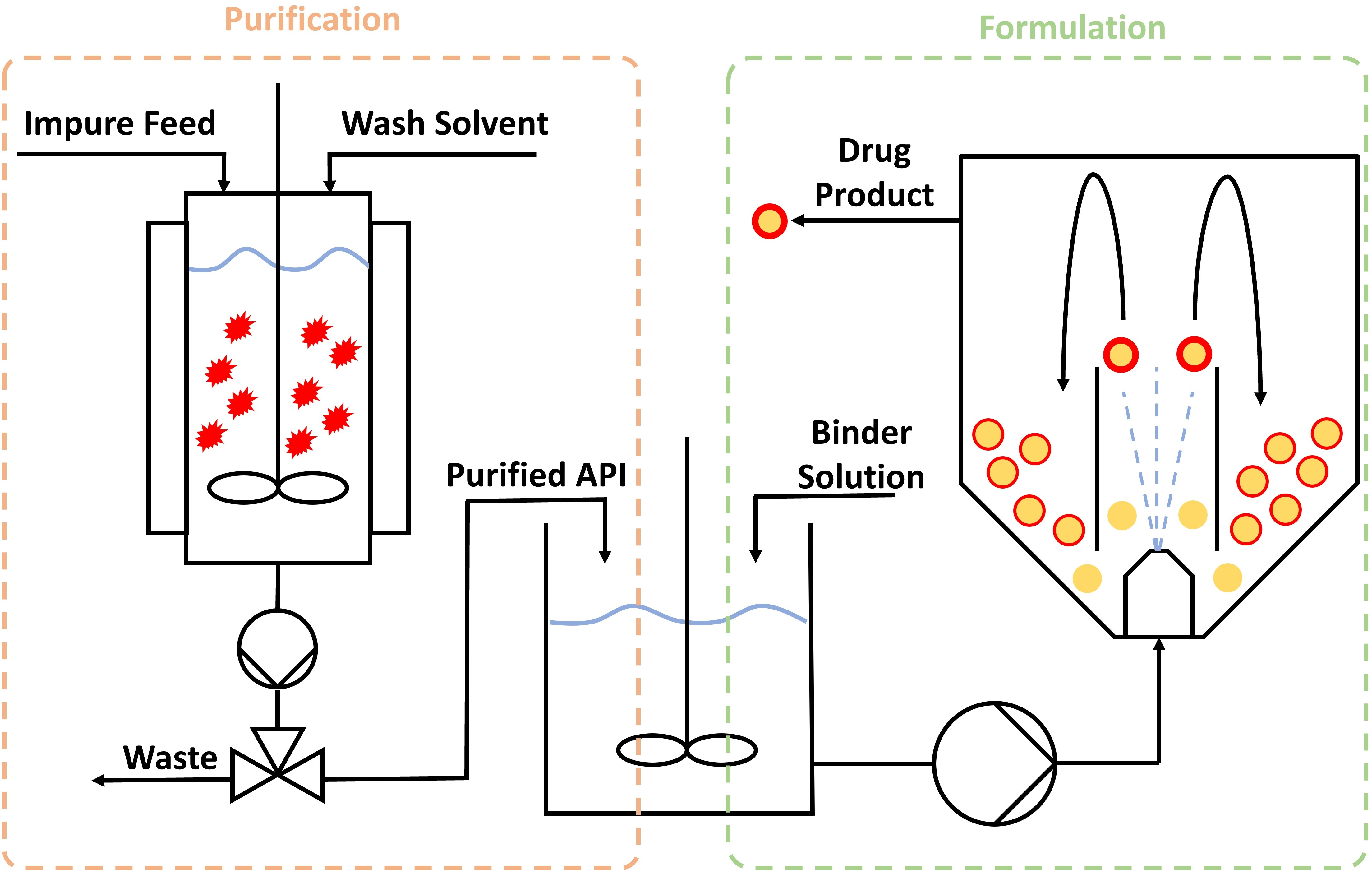
Integrated API and drug product processing enable molecules with high clinical efficacy but poor physicochemical characteristics to be commercialized by direct co-processing with excipients to produce advanced multicomponent intermediates. Furthermore, developing isolation-free frameworks would enable end-to-end continuous processing of drugs. The aim of this work was to purify a model API (sodium ibuprofen) and impurity (ibuprofen ethyl ester) system and then directly process it into a solid-state formulation without isolating a solid API phase. Confined agitated bed crystallization is proposed to purify a liquid stream of impure API from 4% to 0.2% w/w impurity content through periodic or parallelized operations. This stream is combined with a polymer solution in an intermediary tank, enabling the API to be spray coated directly onto microcrystalline cellulose beads. The spray coating process was developed using a Design of Experiments approach, allowing control over the drug loading efficiency and the crystallinity of the API on the beads by altering the process parameters. The DoE study indicated that the solvent volume was the dominant factor controlling the drug loading efficiency, while a combination of factors influenced the crystallinity. The products from the fluidized bed are ideal for processing into final drug products and can subsequently be coated to control drug release.
Continue reading here
About this article: Stocker, M.W.; Harding, M.J.; Todaro, V.; Healy, A.M.; Ferguson, S. Integrated Purification and Formulation of an Active Pharmaceutical Ingredient via Agitated Bed Crystallization and Fluidized Bed Processing. Pharmaceutics 2022, 14, 1058. https://doi.org/10.3390/pharmaceutics14051058
Materials
Sodium ibuprofen (Na Ibu) was purchased from Santa Cruz Biotechnnology (Santa Cruz, CA, USA) with a purity > 99% and free acid ibuprofen was purchased from Kemprotec Limited (Carnforth, UK). Microcrystalline cellulose (MCC) beads (Cellets®) were obtained from Pharmatrans Sanaq AG (Allschwi, Switzerland). The methanol and ethanol used for the coating process were supplied by Corcoran Chemicals (Dublin, Ireland). Polyvinylpyrrolidone (PVP K-25, Mw 31,000 g mol−1) was supplied by BASF (Ludwigshafen, Germany) and hydroxypropyl methylcellulose (HPMC, Pharmacoat® 606, Mw 32,800 g mol−1) was donated by Shin-Etsu Chemical Co. (Tokyo, Japan). HPLC-grade methanol and acetonitrile were purchased from Fisher Scientific (Dublin, Ireland). Monobasic potassium phosphate and sodium hydroxide for buffer preparation were purchased from Sigma Aldrich (Wicklow, Ireland) and Fisher Scientific (Dublin, Ireland), respectively. The ibuprofen ethyl ester impurity (91% purity) was prepared via Fischer–Speier esterification, as previously described.

