Asahi Kasei Contributes to Global Health Care by Supporting Increased Demand for Ceolus™ Microcrystalline Cellulose Pharmaceutical Excipient
NEW YORK–(BUSINESS WIRE)–Forecast expectations for increased global market demand in the excipients industry were confirmed at CPhI this past November where Asahi Kasei, a diversified Japanese multinational company, was met with warm reception for Ceolus™, their ultra-compactible and high-performance microcrystalline cellulose (MCC) excipient. Primarily used in pharmaceuticals as a tablet binder, demand for such excipients has been projected to increase by 5.3% up to US $5.7 billion between 2021 and 2026*1. In addition, the United States and Europe are seeing increased attention for specialty excipients for oral solid dosage form pharmaceuticals as they make up 26% and 25% of the global demand respectively*2. In preparation for this increase of demand, Asahi Kasei announced plans to add a second manufacturing facility in January 2021 and groundbreaking commenced in August. The new facilities are slated for completion in Spring 2023 when its supply capacity will be doubled.
“We were humbled at the attention Ceolus™ received at CPhI this year,” says Kenji Obata, Senior Manager of Functional Additives at Asahi Kasei Europe GmbH. “Despite overall less attendees to the event than average due to the pandemic, the Ceolus™ team saw a strong turnout. We also received a number of inquiries at the event – ranging from sample requests and business cooperation to quotes and more detailed information about Ceolus™ – and we are starting to see how this increase of demand is beginning to affect the industry as a whole. This was a successful event for the Ceolus™ team.”
As more treatments and cures are discovered through advancements in medicine, society has collectively been consuming more pharmaceuticals than ever. Accessibility, versatility, and comfort are becoming key factors for improving the quality of life around the pharmaceuticals we consume. While the market demand has been increasing steadily, there are still many challenges for the industry to make their products more comfortable for the consumers. However, the technology behind Ceolus™ can already provide solutions to many of these problems, and we anticipate further advancements as patient needs evolve.
Significant tablet size reduction
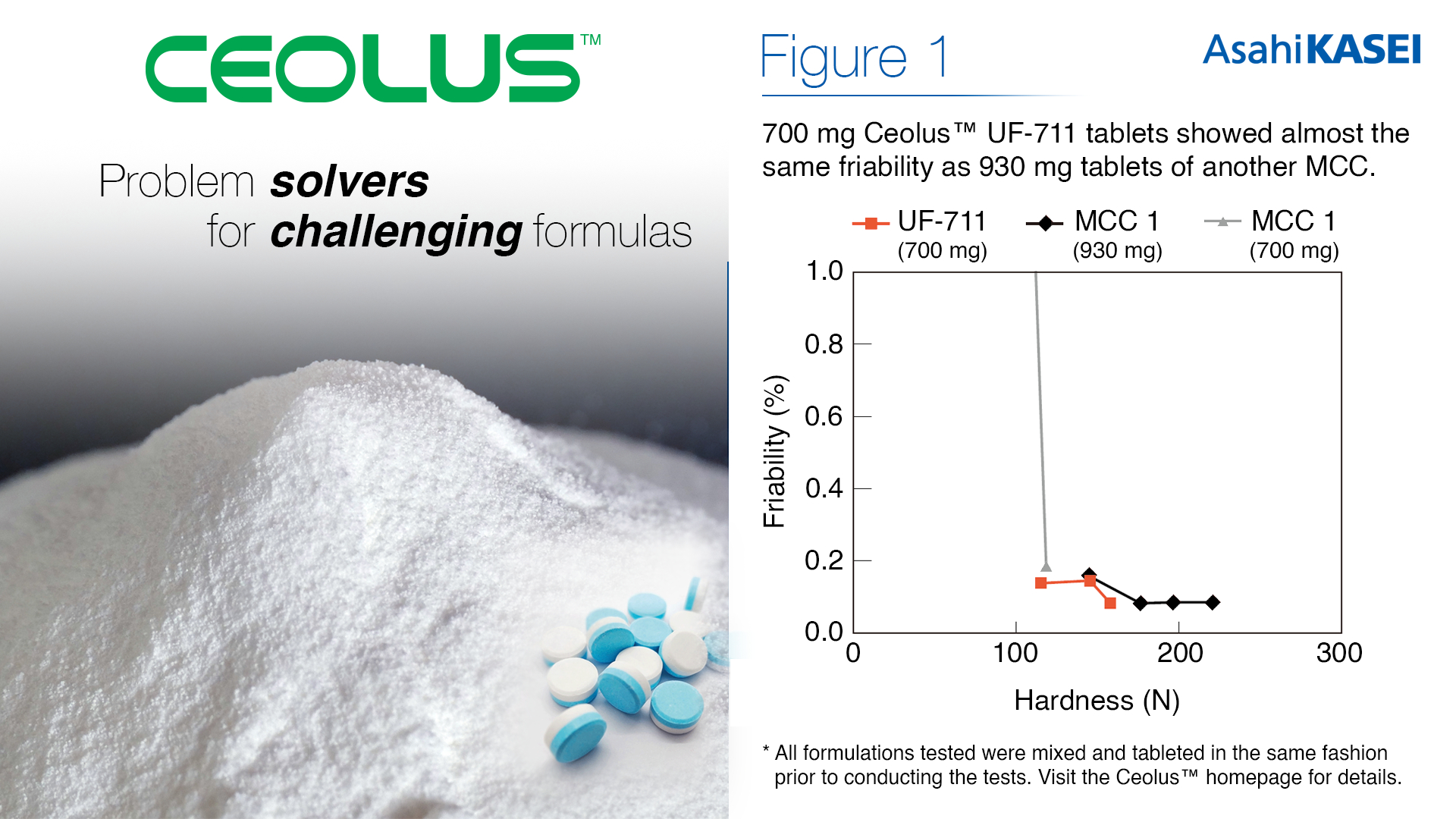
The swallowability of tablets is a comfort factor that poses a challenge to young children, the elderly, or patients with dysphagia. As this pool of patients increases, society has grown to expect smaller tablets for practical and accessible medicine. The high compactibility of Ceolus™ UF-711 allows for sufficient hardness from a smaller amount of MCC used, thus enabling tablet size to be reduced by up to 30% compared to tablets using traditional MCC grades (Figure 1).
Application for nutritional supplements
The challenges of the pandemic have directly led to a heightened awareness for health and an increase in demand for a “cleaner label” of natural supplements. Applying a small amount of the super compactible Ceolus™ KG-1000 to health supplement compounds is a natural solution for maintaining tablet hardness and decreasing friability. In other words, Ceolus™ will safely contain active ingredients without the risk of the tablet splitting or crumbling unintentionally. Asahi Kasei’s study showed that Ceolus™ dissolved the fastest while maintaining less than 1.0% friability regardless of the compression force (Figure 2).
Combination of multiple medications into one tablet
As medicine advances, the average patient begins to consume more and more different types of medication over the course of daily life. When considering drugs for HIV, patients are required to take many different medications daily that can cause a considerable detriment to their quality of life. Some pharmaceutical companies have been successful with formulations that include multiple active pharmaceutical ingredients (APIs) into a single tablet with Ceolus™. This formulation could potentially also combine the APIs for treating HIV or any other diseases that require multiple actives, effectively reducing the number of tablets patients need to take at one time. “Typically, there are a number of challenges that come paired with complex, high-dose formulations that can result in capping, sticking, and high friability,” says Toru Saito, General Manager of Ceolus Overseas Marketing and Sales. “However, Ceolus™ can prevent those issues entirely with only a small amount added. This exemplifies its versatility and capability to improve patients’ quality of life through convenience and expresses exactly why demand for this product has risen so much over recent years. We expect greater volumes of demand as more complex formulas are developed that require the one-of-a-kind performance that Ceolus™ can deliver patients.”
The second Ceolus™ manufacturing facility is set to be completed by Spring 2023, when it will double its supply to the market. Asahi Kasei and the diversified Ceolus™ teams around the world will always continue advancements in pharmaceuticals in harmony with our mission of Creating for Tomorrow.
*1 “Pharmaceutical Excipients Market Global Forecast to 2026,” Markets and Markets, 2021.
*2 “Specialty Excipients for Oral Solid Dosage Form Pharmaceuticals: Global Market Analysis and Opportunities,” Kline & Company, 2020.
Source: Business Wire

