Assessing the utility of in silico tools in early drug development: The case of a pharmaceutically relevant formulation of the prodrug psilocybin
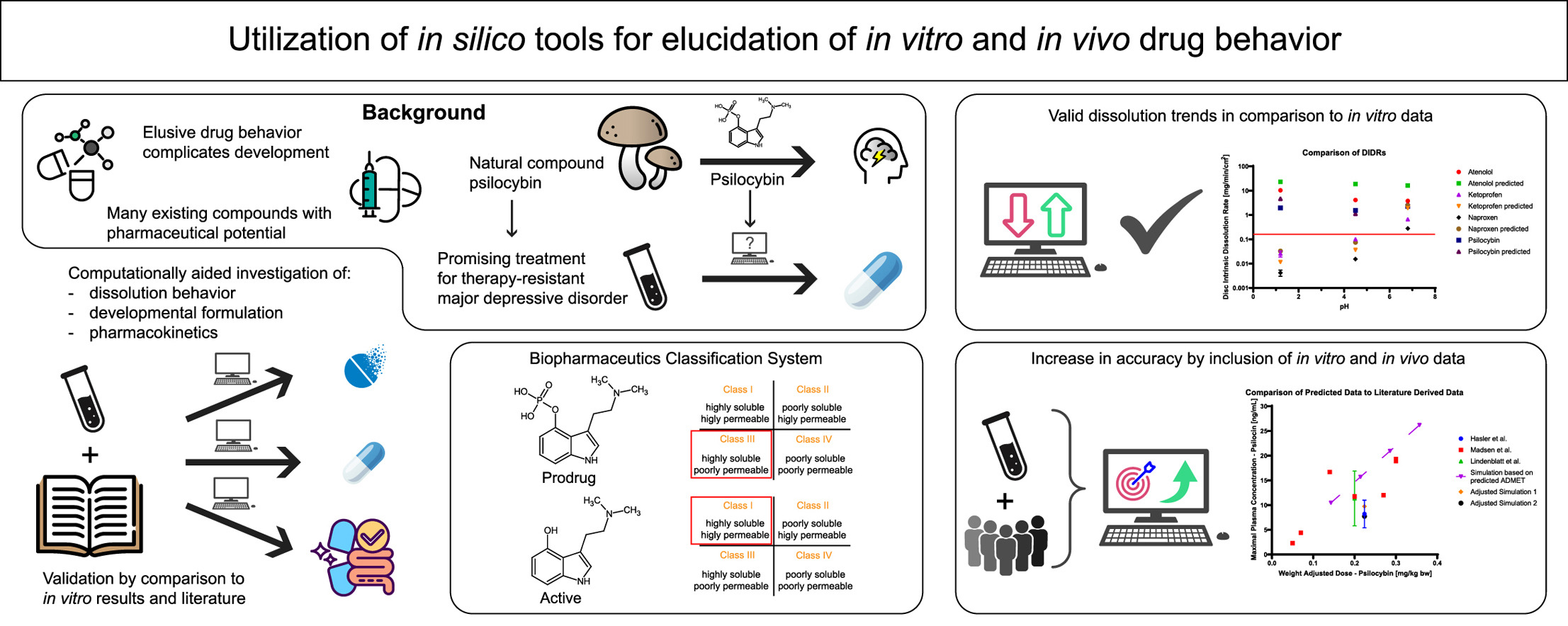
Background: Depressive disorders contribute to an excruciating global mental health burden. Recently, there has been a great shift in interest towards substances with psychedelic potential as treatment for patients with resistant forms of depression, one of these substances being psilocybin. The need to expedite processes within the drug development pipeline and make potential therapy available for patients with treatment-resistant forms of depression is great. Approaches to combine computational and traditional experimental drug development are more consequential due to their potential to shorten development times and aid in setting product specifications. To date, the full extent to which in silico methods for early drug development yield valid and robust data which can be directly used for translation into safe and effective pharmaceutical products, remains unknown, especially for prodrug containing formulations.
Methods: Herein we explore the robustness and applicability of in silico generated data for the aid of experimental drug development by directly comparing computationally generated data to experimentally and literature derived data. Furthermore, by utilizing different computational approaches, we investigate to which extent these computational models can by utilized to create a novel capsule containing psilocybin, having in mind that psilocybin is a prodrug and psilocin is the active.
Results: In silico models provided valid data including intrinsic dissolution and capsule dissolution behavior, but were not able to predict stability issues. Simulations of the expected in vivo performance showed deviations from previously published clinical studies. Significant improvements in predictive accuracy were made by implementing physicochemical surface pH models and entering in vitro and in vivo data.
Conclusions: Computational models provide a valuable resource to aid in the planning and implementation of experimental drug product development. Prodrug and active were simulated for their in vitro and in vivo behavior. Even though the results appear to be robust, these models exhibit potential for improvement in predictive accuracy, and thus, applicability of computationally generated data for drug development. Moreover, the models are yet unable to perform a completely accurate drug development process solely supported by in silico data. Lastly, we were able to report psilocybin as a BCS class III drug and psilocin as a BCS class I drug in terms of a provisional classification supported by our data.
Read more about the use of in silico tools in early drug development
Materials
The drugs ketoprofen, naproxen, and atenolol were obtained from Medisca Inc. (St-Laurent, QC, Canada); psilocybin was synthesized by the Drug Development and Innovation Centre (Edmonton, AB, Canada). PROSOLV® EASYtab SP was used for compounding psilocybin and was derived from JRS PHARMA GmbH & Co. KG (Rosenberg, Germany). Buffers were prepared according to the United States Pharmacopeia (USP) [14].
Luca Maurice Richter, Jozef Al-Gousous, Gabriel Lima Barros de Araujo, Neal M. Davies, Raimar Löbenberg,
Assessing the utility of in silico tools in early drug development: The case of a pharmaceutically relevant formulation of the prodrug psilocybin, Journal of Drug Delivery Science and Technology, 2024, 105305, ISSN 1773-2247, https://doi.org/10.1016/j.jddst.2023.105305.
Read more on “Lubricants” here:


