Assessment of blend uniformity in a stream sampler device using Raman spectroscopy
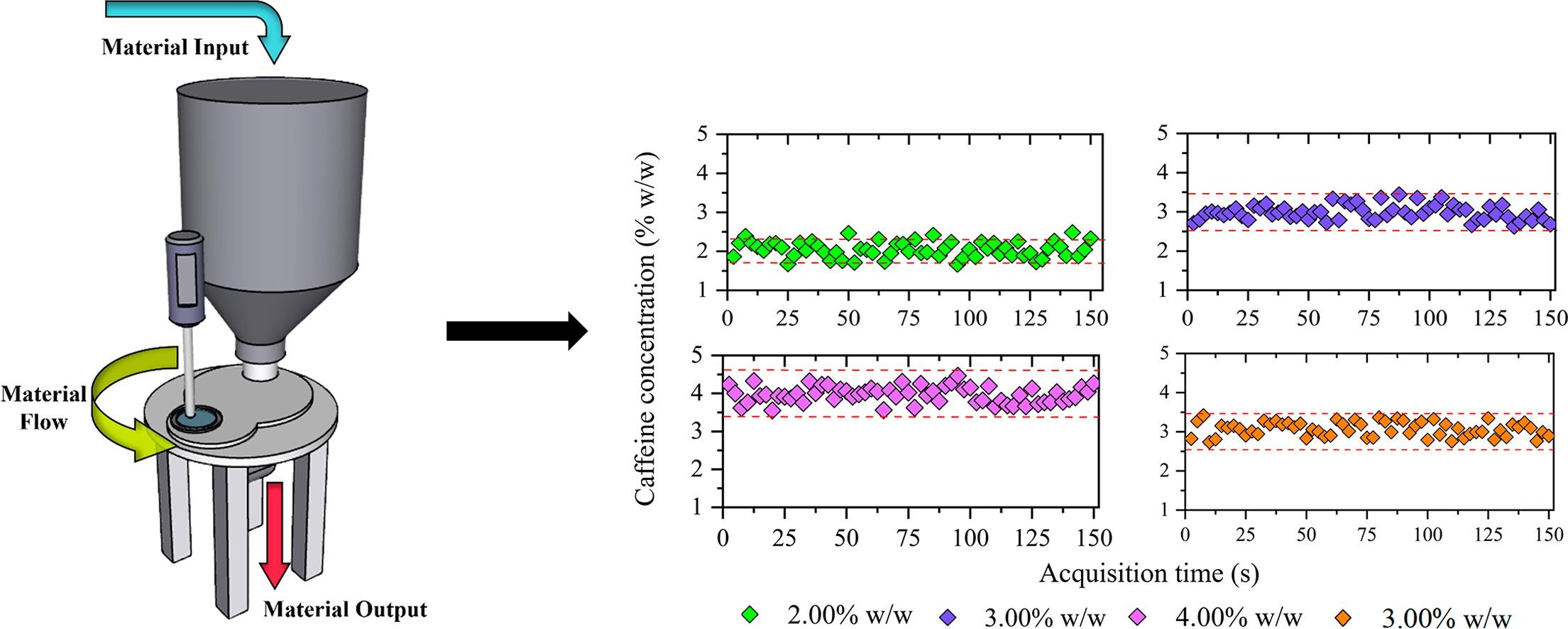
This study describes the first implementation of Raman spectrometer in a stream sampler for the in-line monitoring of low drug concentration in poor flowability powder blends. Raman spectra were continuously acquired as the powder blends flowed through the stream sampler operating with a paddle wheel speed of 10 RPM and used to develop the calibration models. A calibration model was developed to quantify caffeine concentration from 1.50 to 4.50% w/w using Partial Least Squares Regression (PLS-R). Three test set blends were used to assess the prediction errors of the calibration model. Caffeine concentration was predicted for the test set blends with a root mean square error of prediction of 0.21% w/w and a low bias of −0.03% w/w. The calibration model showed good prediction performance with an estimated sample mass of 83 mg. Variographic analysis demonstrated the low process variance of the real-time spectral acquisition through minimum practical error and sill values. The results showed the ability of the Raman spectrometer coupled with the stream sampler to monitor low drug concentration for poor flowability blends.
Introduction
Section 211.110 of the current Good Manufacturing Practices (cGMPs) states that solid oral dosage forms manufacturers should perform blend uniformity analysis to assess the adequacy of mixing to ensure the correct amount of active pharmaceutical ingredient (API) is delivered to the patients from each unit dosage (Food and Drug Administration, 2001). A traditional batch methodology for determining blend uniformity is performed by stopping the batch mixer and inserting a sample thief to manually acquire samples in preselected locations (Muzzio et al., 1997). The insertion of the sampling thief may cause segregation and alter the distribution of the particles in the powder blend leading to changes in the composition of the samples collected, raising the uncertainty of the decision regarding the batch (Esbensen et al., 2016). The acquired samples are analyzed by conventional analytical techniques such as ultraviolet–visible spectrophotometry or high-performance liquid chromatography (Muzzio et al., 1997), which are destructive, time-consuming, and not suitable for real-time monitor and control of blend uniformity. Near-infrared (NIR) spectroscopy and Raman spectroscopy have been used as an alternative to the sampling thief (Colón et al., 2014). These vibrational techniques provide a non-destructive analysis without sample preparation and allow the collection of a high number of samples in a short time (de Beer et al., 2011).
The success of implementing NIR and Raman spectroscopy in real-time monitoring of blend uniformity for flowing powders depends, among other factors, on the design of the sampling interface, which must be optimized to ensure representative sampling (Sierra-Vega et al., 2020a, U.S Department of Health and Human Services, 2021). A chute in the discharge of the continuous blender is a popular sampling interface to analyze the powder concentration. Different chute designs have been presented for the installation of the spectroscopic probe (Colón et al., 2014, Sierra-Vega et al., 2019, Vanarase et al., 2010). However, studies reported the presence of segregation and non-reproducible powder flow behavior within the chutes (Vanarase et al., 2013, Vargas et al., 2017), as well as high sampling and analytical errors in the drug concentration evaluation (Sierra-Vega et al., 2019). These negative features significantly affect the determination of blend quality. A stream sampler device was developed as an alternative to chutes and to improve the sampling for blend uniformity (Romañach and Méndez, 2019).
The stream sampler has proved to be a suitable sampling device to monitor blend uniformity in flowing powder blends using NIR spectroscopy (Alvarado-Hernández et al., 2020, Sierra-Vega et al., 2020b, 2020a). Studies in the stream sampler have been conducted considering changes in both API concentrations and physical properties of powder blends. As a first approach, this sampling interface quantified 15% w/w caffeine, providing a consistent mass flow of 30 kg/h without affecting the composition or properties of free-flowing powder blends. Also, the stream sampler has been used in the real-time monitoring of 0.52 and 4.52% w/w drug concentration (Sierra-Vega et al., 2020a). The developed NIR models were robust at changes of ± 10% of the target paddle wheel speed (10 RPM). The stream sampler also was characterized using low-dose cohesive formulations (Sierra-Vega et al., 2020b). Results showed that an increment in the cohesion of powder blends increases sampling and analytical errors. In these three studies, the stream sampler presented successful results using a benchtop NIR spectrometer with a 10 mm illuminated spot diameter (Sierra-Vega et al., 2020b). NIR spectroscopy has played an essential role in the characterization of the sampler device. This study extends the evaluation of the performance of the stream sampler using Raman spectroscopy.
The development of new portable Raman technologies has increased its interest as a PAT tool for real-time monitoring blend uniformity (Löbenberg and Bou-Chacra, 2020). Portable Raman spectrometers offer an easy flexibility and versatile interface with a smaller size and weight for easier installation on-site. A 6 mm spot diameter probe portable Raman spectrometer was previously used to develop an offline method and transfer it to the manufacturing line to monitor an 8% w/w formulation at the tablet press feed frame (Li et al., 2018). The in-line Raman method analyzed approximately 1400 mg per spectrum, obtaining RMSEP for the test set of 1.64% w/w (Li et al., 2018). Raman and NIR spectroscopy also were used in combination with an extended iterative optimization technique (EIOT) to in-line monitor API concentration in the tablet press feed frame from 1 to 8% w/w using three test set blends (1, 3, and 8% w/w) (Harms et al., 2019). A 3 mm spot diameter Raman probe was used to monitor the drug concentration providing satisfactory outcomes. Results between the two techniques were compared, demonstrating that Raman spectroscopy showed less day-to-day variability and may detect a lower API concentration in the powder blends than NIR spectroscopy (Harms et al., 2019).
This research paper provides the first application of the stream sampler using a portable Raman spectrometer to monitor low drug concentration in flowing powder blends with poor flowability. The main objective was the development of a Raman calibration model for real-time monitoring of caffeine concentrations between 1.5 and 4.5% w/w. A MarqMetrix® Raman All-In-One spectrometer (MarqMetrix, Seattle, WA, USA) equipped with 1.8 mm spot diameter Raman BallProbe® was used for spectral acquisition. A lower spot diameter implies a low sample mass analyzed when the spectral acquisition is done, providing a more challenging development of spectroscopic calibration models. The PLS calibration model was evaluated in terms of accuracy, repeatability, and intermediate precision. The final part of the study entails the use of variographic analysis to characterize the process variation, and the sampling and analytical errors.
Read more
Materials
Anhydrous caffeine (NutriVita®) was used as the model API. The main excipients in the formulations were lactose monohydrate (Tablettose® 100, Ph. Eur., USP-NF/JP, Molkerei) and microcrystalline cellulose (MCC, Avicel® PH-302, NF, Ph. Eur., JP, FMC Corporation). Magnesium stearate (MgSt, N.F. non-Bovine, Tyco Healthcare/Mallinckrodt, St. Louis, Missouri, MO, USA) was used as a lubricant, and colloidal silicon dioxide (SiO2) from ACROS Organics as a glidant.
Raúl S. Rangel-Gil, Nobel O. Sierra-Vega, Rodolfo J. Romañach, Rafael Méndez, Assessment of blend uniformity in a stream sampler device using Raman spectroscopy, International Journal of Pharmaceutics, Volume 639, 2023, 122934, ISSN 0378-5173, https://doi.org/10.1016/j.ijpharm.2023.122934.
Read more on Magnesium Stearate as a pharmaceutical excipient here:


