Chitosan and Anionic Solubility Enhancer Sulfobutylether-β-Cyclodextrin-Based Nanoparticles as Dexamethasone Ophthalmic Delivery System for Anti-Inflammatory Therapy
Abstract
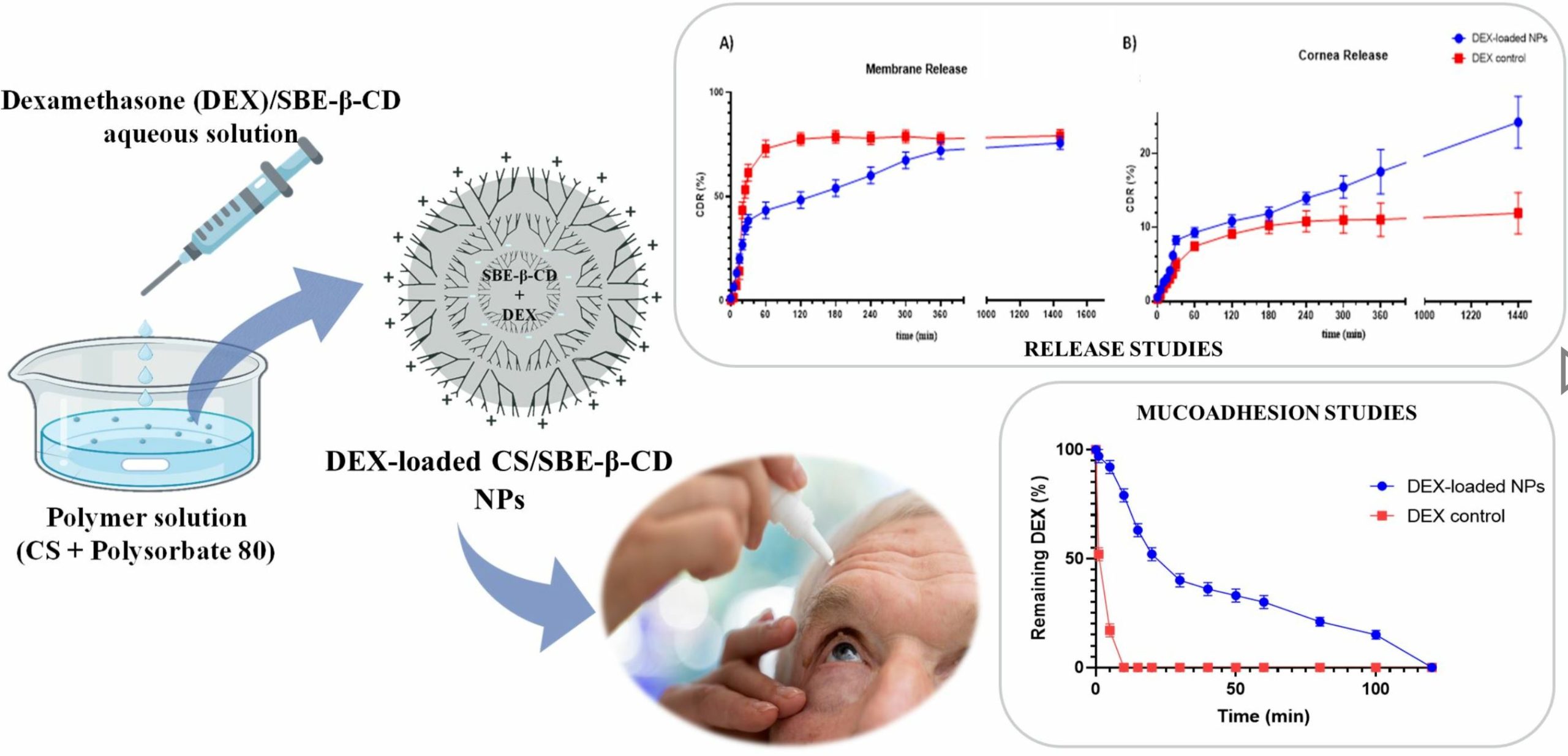
Cataract surgery interventions are constantly increasing, particularly among adult and elderly patients. This type of surgery can lead to inflammatory states of the ocular anterior segment (AS), usually healed via postoperative treatment with dexamethasone (DEX)-containing eye drops. The application of eye drops is challenging due to the high number of daily administrations. In this study, mucoadhesive nanoparticles (NPs) were formulated to improve the residence time of DEX on the corneal mucosa, enhancing the drug’s solubility and bioavailability. The NPs were generated using an ionotropic gelation technique, exploiting the interaction between the cationic group of chitosan (CS) and the anionic group of sulfobutylether-β-cyclodextrin (SBE-β-CD). The formation of the inclusion complex and its stoichiometry were studied through phase solubility studies, Job’s plot method, and Bi-directional transport studies on MDCKII-MDR1. The obtained NPs showed good chemical and physical characteristics suitable for drug loading and subsequent testing on animal mucosa. The DEX-loaded CS/SBE-β-CD NPs exhibited a prolonged residence time on animal mucosa and demonstrated enhanced drug permeability through the corneal membrane, showing a sustained release profile. The developed NPs posed no irritation or toxicity concerns upon local administration, making them an optimal and innovative drug delivery system for inflammatory AS diseases treatment.
Introduction
Cataracts are one of the world’s major eye diseases, characterized by a gradual opacification of the eye lens, which can lead to complete blindness of the patient [1], and it affects between 11.8% and 18.8% of the world’s population [2]. Its incidence increases with age, ranging from 3.9% among 55–64-year-olds to 92.6% among those 80 years and older [3]. Many techniques have been used to treat the disease, including intraocular lens (IOL) placement [4], femtosecond laser-assisted surgery (FLACS) [5], and other pharmacological techniques such as the use of lanosterol to recover lens transparency [6]. To date, despite pharmacological techniques such as lanosterol and vitamin D or compounds possessing antioxidant and free-radical scavenging activity having shown promising potential in experimental studies, none of these drug candidates have translated into FDA-approved anti-cataract eye drops or remedies capable of preventing or treating cataracts in humans [7]. Therefore, surgery remains the main and most effective option for the treatment of this disease. However, surgical intervention can easily lead to an acute state of inflammation of the ocular anterior segment (AS) on postoperative days, which occurs with diffuse corneal edema and results in diffuse corneal endothelial damage [8]. Furthermore, when treating patients of different age groups, changes in the involved ocular tissues that may affect the incidence of the inflammatory state must also be considered. Compared to other ocular structures, the cornea does not show significant changes with normal ageing. However, clinically evident tissue changes are present that are asymptomatic and do not affect vision, making them difficult to detect. The aged cornea becomes more susceptible to infection due to a decreased ability to withstand a variety of physiological stresses [9]. In fact, the thickness of the cornea tends to flatten out with age, with a conspicuous loss of corneal endothelial cell density in an advanced age, thus leading to an increased risk of incurring inflammatory and infectious states following traumatic events such as cataract surgery [10]. Considering the extremely high number of such surgeries worldwide, perioperative organization and prophylaxis are important to improve the recovery time and quality of the patient’s response to surgery [11]. A reduction in AS inflammation resulting from surgical practice contributes to improved final visual outcomes in patients recovering from surgery [12].
Dexamethasone (DEX) is commonly used in the clinical treatment of patients who have undergone cataract surgery [13]. DEX is a fluorinated synthetic corticosteroid and is currently the most potent synthetic analog of cortisol [14]. In the treatment of ocular AS, DEX prevents and treats retinal vein occlusion and noninfectious uveitis, greatly reducing the postoperative inflammatory phenomenon [15,16].
Most commercially available medications for the treatment of inflammatory states in AS are provided as eye drops [17], which include DEX-loaded Drug Delivery Systems (DDS) [18]. Indeed, apart from instances of DEX being loaded into solid DDS like tablets or intravitreal implants [19], the most commonly used dosage forms currently available on the market are eye drops in which the drug is in suspension, either alone or in the presence of other active ingredients (e.g., levofloxacin, tobramycin, netilmicin). Formulations to date on the market have a 0.1% w/v of DEX, and the usual dose of DEX-based eye drops is one drop per eye repeated every 4 h. The administration of eye drops formulations is particularly challenging [20] due to the presence of static ocular barriers, such as anatomical (cornea, sclera, retina), haemato-aqueous, and haemato-retinal barriers.
Moreover, the presence of dynamic barriers, such as the nasolacrimal duct, blinking reflex, and the low receiving volume of the conjunctival sac (20–50 µL), greatly reduce the bioavailability of drugs at the ocular AS [21]. In addition, adherence to therapy remains a critical issue, especially in the adult and elderly populations, who constitute the largest proportion of those exposed to this type of surgery [22]. The use of eye drops can represent an obstacle for these patients, given the difficulty in instilling the drops in multiple and repeated administrations at different times of the day. Thus, there is a need to formulate a safe, manageable, and effective product that can ensure the resolution of inflammation and optimal adherence to therapy [20].
Nanoparticles (NPs) have proven useful in overcoming static and dynamic ocular barriers by improving the pharmacokinetic properties and bioavailability of the drug and protecting it from physical, chemical, and biological degradation phenomena [23]. The advantages of nanosystems used as ophthalmic drug delivery systems also include increased permeability and bioavailability of the drug, increased retention of the drug on the surface of the eye, and improved interaction with the mucous membrane of the cornea [24]. In fact, smaller particles, such as nanoparticles, are well tolerated and can exhibit adhesive characteristics which are responsible for the prolonged contact time of a drug with the ocular tissue and its increased bioavailability [25]. In addition, the small size of NPs allows for the crossing of physical ocular barriers and facilitates drug release at the target site [26].
CS is a linear polysaccharide produced by the deacetylation of chitin and is commonly used in the formulation of CS-based NPs for medical purposes [27]. The polycationic nature of chitosan ensures strong ionic interactions with negatively charged components present on the surface of the biological mucosa, such as sialic acid [28], making CS a strongly mucoadhesive polymer [29].
However, DEX is known to be a substrate of P-glycoprotein (P-gp) [30] and induces P-gp overexpression in human retinal pigment epithelial cells after prolonged instillation [31]. P-gp is an efflux pump that hinders drug adsorption, and its overexpression is often present in inflamed tissues and has been reported to be associated with chemoresistance and poor prognosis in several ocular malignancies [32]. Thus, overexpression of P-gp in ocular tissues would result in the rapid clearance of DEX from the ocular surface if it were directly instilled as a suspension in an eye drop. Moreover, lipophilic molecules such as DEX (LogP = 1.83) often present a persistent challenge due to their low aqueous solubility which reduces their bioavailability and limits their therapeutic use [33].
In previous studies, it has been shown that cyclodextrins are poor substrates for P-gp [34], and, specifically, SBE-β-CD causes a direct inhibition of P-gp ATPase [35]. Moreover, by establishing inclusion complexes with hydrophobic drug molecules, the CDs are frequently used in DDS to improve the water solubility of poorly soluble drugs [36,37]. SBE-β-CD is a polyanionic derivative of β-CD characterized by the presence of a sodium sulfonate salt separated from the lipophilic cavity by a butyl ether spacer group [38]. The negative-charge repulsive forces of the terminal groups extend the cavity of this cyclodextrin, making it preferable for its stronger drug binding [39] and resulting in it being exploitable for interaction with polycationic chains in the NPs production process via ionotropic gelation. The inclusion complex formation has been used to enhance the stability of DEX in the ocular environment and permeability through the cornea owing to the higher solubility in the lacrimal fluids and also to the inhibition of P-gp exerted by SBE-β-CD [40]. Moreover, DEX/cyclodextrins inclusion complexes in association with CS have exhibited improved drug solubilization, enhanced mucoadhesive characteristics, and low toxicity in ophthalmic use [41].
The aim of this study was to develop novel nanotechnology-based eye drops for ophthalmic delivery of DEX, generating, for the first time, an SBE-β-CD/DEX inclusion complex-based formulation eligible as novel anti-inflammatory therapy post cataract surgery. In this work, a detailed investigation was carried out on the inclusion complex formation pathways between SBE-β-CD and DEX, with a focus on drug-CD interactions. A detailed characterization of the chemical–physical properties and mucoadhesive characteristics of NPs was carried out. In addition, the drug release and permeability were evaluated. These aspects were exploited to increase the residence time of the drug on the ocular mucosal surface, achieving a controlled release of the drug and decreasing the number of daily administrations of the eye drops when compared to commercially available formulations.
Download the full article as PDF here: Chitosan and Anionic Solubility Enhancer Sulfobutylether-β-Cyclodextrin-Based Nanoparticles as Dexamethasone Ophthalmic Delivery System for Anti-Inflammatory Therapy
or read it here
Materials
Low-molecular-weight chitosan (139.9 kDa, degree of deacetylation: 0.9), medium-molecular-weight chitosan (445.8 kDa, degree of deacetylation: 0.9), high-molecular-weight chitosan (749.4 kDa, degree of deacetylation: 0.9), and oligomer chitosan (13.49 kDa, degree of deacetylation: 0.8) were kindly donated from Primex EHF (Siglufjordur, Iceland). Sulfobutylether-β-Cyclodextrin sodium salt (Mw 2163, degree of substitution 6.5) (Captisol®) was purchased from CyDex Pharmaceuticals (Lenexa, KS, USA). Hydroxypropyl-β-cyclodextrin and dexamethasone were purchased from Farmalabor Srl (Canosa di Puglia, Italy). Calcium chloride dihydrate, polysorbate 80, potassium bromide, potassium chloride, potassium phosphate monobasic, sodium bicarbonate, sodium chloride, and sodium phosphate dibasic were purchased from Sigma-Aldrich–Merck Italy (Milano, Italy). MilliQ water used in the assays was obtained through a Milli-Q instrument by Millipore Sigma (Burlington, MA, USA). The human MDR1-transfected MDCKII cell line (MDCKII- MDR1) was obtained from Dr. Piet Borst (Netherlands Cancer Institute, Amsterdam, The Netherlands).
Racaniello, G.F.; Balenzano, G.; Arduino, I.; Iacobazzi, R.M.; Lopalco, A.; Lopedota, A.A.; Sigurdsson, H.H.; Denora, N. Chitosan and Anionic Solubility Enhancer Sulfobutylether-β-Cyclodextrin-Based Nanoparticles as Dexamethasone Ophthalmic Delivery System for Anti-Inflammatory Therapy. Pharmaceutics 2024, 16, 277. https://doi.org/10.3390/pharmaceutics16020277

