Improved Bioavailability of Poorly Water-Soluble Drug by Targeting Increased Absorption through Solubility Enhancement and Precipitation Inhibition
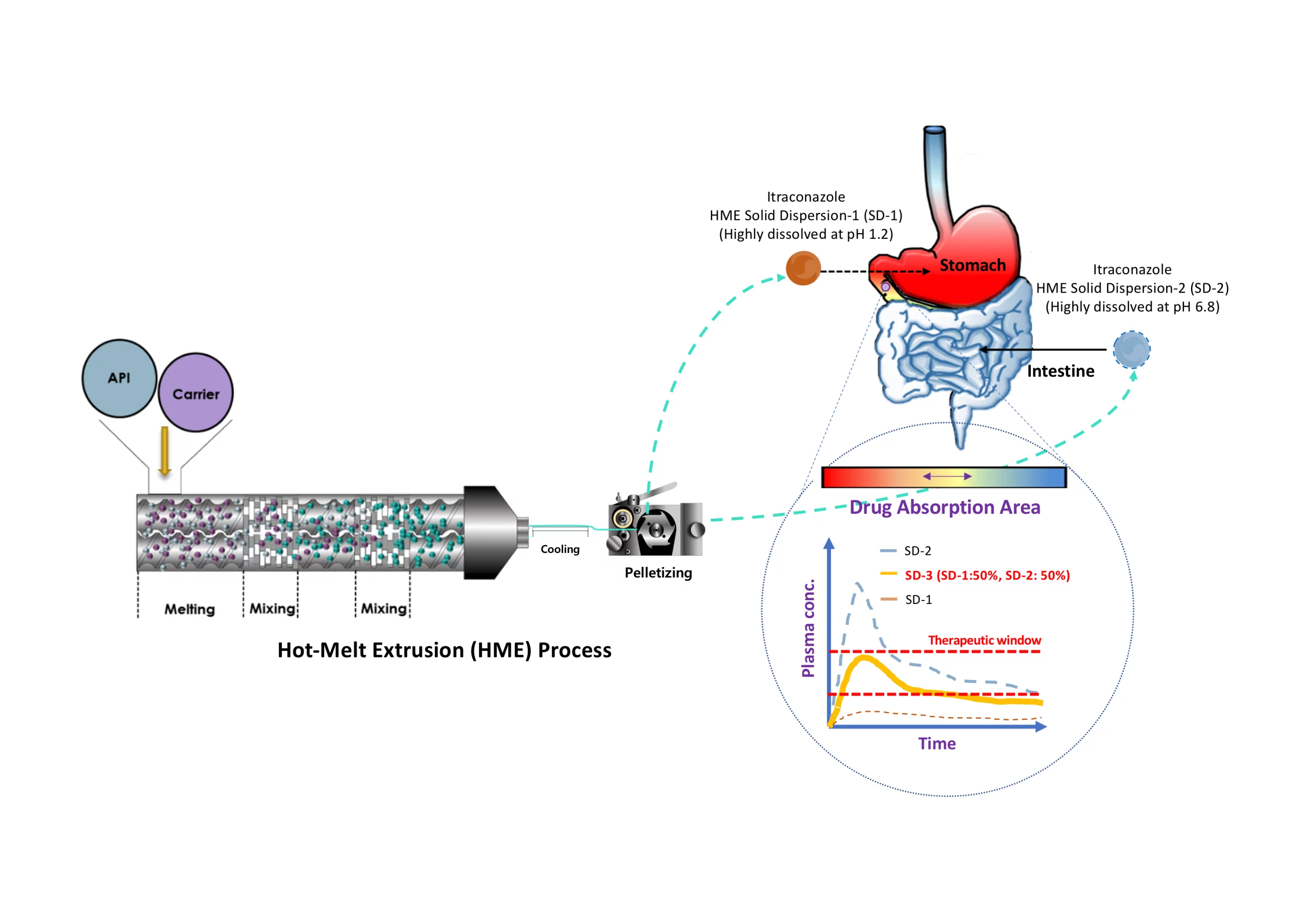
Itraconazole (ITZ) is a class II drug according to the biopharmaceutical classification system. Its solubility is pH 3-dependent, and it is poorly water-soluble. Its pKa is 3.7, which makes it a weak base drug. The aim of this study was to prepare solid dispersion (SD) pellets to enhance the release of ITZ into the gastrointestinal environment using hot-melt extrusion (HME) technology and a pelletizer. The pellets were then filled into capsules and evaluated in vitro and in vivo. The ITZ changed from a crystalline state to an amorphous state during the HME process, as determined using DSC and PXRD.
In addition, its release into the gastrointestinal tract was enhanced, as was the level of ITZ recrystallization, which was lower than the marketed drug (Sporanox®), as assessed using an in vitro method. In the in vivo study that was carried out in rats, the AUC0–48h of the commercial formulation, Sporanox®, was 1073.9 ± 314.7 ng·h·mL−1, and the bioavailability of the SD pellet (2969.7 ± 720.6 ng·h·mL−1) was three-fold higher than that of Sporanox® (*** p < 0.001). The results of the in vivo test in beagle dogs revealed that the AUC0–24h of the SD-1 pellet (which was designed to enhance drug release into gastric fluids) was 3.37 ± 3.28 μg·h·mL−1 and that of the SD-2 pellet (which was designed to enhance drug release in intestinal fluids) was 7.50 ± 4.50 μg·h·mL−1.
The AUC of the SD-2 pellet was 2.2 times higher than that of the SD-1 pellet. Based on pharmacokinetic data, ITZ would exist in a supersaturated state in the area of drug absorption. These results indicated that the absorption area is critical for improving the bioavailability of ITZ. Consequently, the bioavailability of ITZ could be improved by inhibiting precipitation in the absorption area.
Download the full article as a PDF here or read it here
Materials: ITZ (USP grade) was purchased from SK Chemical (Seoul, Korea). PVA (Parteck® MXP, average molecular weight: ~32,000) was supplied by Merck KgaA (Darmstadt, Germany). Triethyl citrate (≥99%, molecular weight: 276.28) was purchased from Sigma-Aldrich (Seoul, Korea). Polyvinyl caprolactam-polyvinyl acetate-polyethylene glycol graft copolymer (PCL-PVAc-PEG, Soluplus®, average molecular weight: ~118,000) was supplied by BASF (Ludwigshafen, Germany). HPMCP-HP55 (hypromellose phthalate, average molecular weight: ~45,580) was obtained from Shin-Etsu Chemical (Tokyo, Japan). All chemicals and solvents used were of analytical grade.
Article information: Lee, J.-H.; Park, C.; Weon, K.-Y.; Kang, C.-Y.; Lee, B.-J.; Park, J.-B. Improved Bioavailability of Poorly Water-Soluble Drug by Targeting Increased Absorption through Solubility Enhancement and Precipitation Inhibition. Pharmaceuticals 2021, 14, 1255. https://doi.org/10.3390/ph14121255

