Part III of the series – Encapsulating cannabinoids in lipid-based nanoparticles: THC vs. CBC
Enhancing cannabinoids’ therapeutic efficacy
In our previous technical note, we reported on the encapsulation of CBD vs THC (1) into lipid-based nanoparticles. The work here-in continues on that theme in terms of studying the differences in encapsulating different cannabinoids into the same lipid-based drug delivery system specifically developed for one of the major cannabinoids, THC. The overarching hypothesis in our series of experiments is that the similarities in cannabinoids could enable similar results in terms of nanoparticle stability and encapsulation efficiency. But as we note in the various comparisons developed in this series, small changes in structure can have interesting outcomes. Let us take a closer look here at Tetrahydrocannabinol (THC) and cannabichromene (CBC).
Cannabinoids have a long history in medicine given their therapeutic effects (2). However, due to their lipophilic nature, they are poorly absorbed by the digestive system which hinders their potential as therapeutics (3).
Nanoparticles offer a solution to this problem. By encapsulating these hydrophobic molecules into water miscible vehicles, they can be better absorbed by the digestive system, thus enhancing their therapeutic effect (4).
At Ascension Sciences, we have explored different types of nanoparticles to encapsulate cannabinoids. Specifically, we developed multiple lipid-based formulations that have been successfully optimized for the encapsulation of THC. One of the next steps in our exploration was to use these optimized parameters to encapsulate CBC, a non-psychotropic cannabinoid, into three types of lipid-based nanoparticles: emulsions, liposomes and solid-lipid nanoparticles (SLNPs) – Figure 1.
 Figure 1: Lipid-based nanoparticles
Figure 1: Lipid-based nanoparticles
THC vs. CBC
This technical note presents the comparison between the encapsulation of THC vs. CBC using formulation parameters that were previously optimized for THC. The goal of the study was to provide insights into how the formulation optimized for one cannabinoid translates into another, and where one lead formulation concept can become the starting point for an alternate active ingredient.
OVERVIEW
Tetrahydrocannabinol (THC) and cannabichromene (CBC), along with cannabinol and cannabidiol, are the most abundant naturally occurring cannabinoids (5, 6). As seen in Fig. 2, these two cannabinoids have similar molecular structure. However, small structural differences result in THC and CBC having different chemical properties and effects on our body’s endocannabinoid system.
As mentioned in the previous article, THC is the primary psychoactive compound found in the cannabis plant and works as a partial agonist of cannabinoid receptors (CB1 and CB2) and has effects on emotion, pain, digestion, and appetite (7, 8). CBC, a non-psychoactive component in marijuana (9) has been discovered to not interact with CB1 receptors but to interact with transient receptor potential (TRP) cation channels, which inhibit endocannabinoids inactivation and are linked to pain and inflammation (10, 11, 12, 13). CBC, like other natural plant products, can inhibit the cellular reuptake of endocannabinoids (14). While CBC is one of the prominent non-psychoactive cannabinoids having a range of benefits, there remains a lack of basic insights about its pharmacology. Some potential uses of CBC include: antibacterial effects (15), anti-inflammatory action and pain relief (16), anti-depressant effects (17), anti-cancer (18), anti-acne activities (19), and promotion of cell growth (20).
 Figure 2: Molecular structure of THC and CBC
Figure 2: Molecular structure of THC and CBC
In this specific study, we compared the encapsulation efficiency and nanoparticle stability of each cannabinoid formulation. Encapsulation efficiency is the amount of cannabinoid that is encapsulated into the nanoparticle upon formulation. Nanoparticle stability is determined by measuring the particle size and polydispersity index (PDI) every 7 days for a month of storage at different conditions to monitor any significant changes in the properties of the particles. A stable nanoparticle does not significantly change in size and PDI remains below 0.2, meaning that the particle population is uniform in size and there is no aggregation of the particles over time. Lastly, we determined the cannabinoid retention, which is the amount of cannabinoid that remained encapsulated inside the nanoparticles after 35 days of storage.
METHODS
The formulation parameters for each nanoparticle type can be found in Table 1. Each formulation type was used on either THC or CBC using the same formulation parameters.
All of the nanoparticle formulations were prepared using a low energy approach achieved by the NanoAssemblr Benchtop microfluidic instrument from Precision Nanosystems.
| Emulsion | Liposome | SLNP | |
| Lipid composition | TweenⓇ 80 : SpanⓇ 80 : Hemp Seed Oil | POPC : Chol : DSPE-PEG2000 | Chol : POPC : DSPE-PEG2000 |
| Organic solvent | Ethanol | ||
| Aqueous solvent | Deionized water | PBS pH 7.4 | |
| Solvent removal | Dialysis in aqueous media | ||
| Lipid:Cannabinoid | 10:1 | ||
Table 1: Formulation parameters for the different types of nanoparticles
RESULTS
Encapsulation efficiency (EE%)
 * SLNP: Solid-Lipid Nanoparticle
* SLNP: Solid-Lipid Nanoparticle
Figure 3: Encapsulation efficiency of THC and CBC in each type of nanoparticle
| Type of nanoparticle | THC concentration, mg/mL | CBC concentration, mg/mL |
| Liposome | 0.97 | 0.56 |
| Emulsion | 3.89 | 3.47 |
| SLNP | 0.30 | 0.31 |
Table 2: Encapsulated cannabinoid concentration
* THC solubility in water: 2.8 ug/mL (21)
*CBC solubility in water: 1.54 ug/mL (22)
- THC and CBC both showed similar EE% in the nanoemulsion.
- Comparing the two cannabinoids, liposomes showed a difference in EE% between the two. THC liposomes showed greater EE% of 72% compared to 45% for CBC liposomes.
- SLNPs showed similar EE% between the two cannabinoids and the highest EE% between the different nanoparticle types for both THC and CBC.
Nanoparticle stability over 35 days of storage at 4°C
 SLNP: Solid-Lipid Nanoparticle
SLNP: Solid-Lipid Nanoparticle
Figure 4: Stability of the nanoparticles over 35 days of storage at 4°C.
- THC and CBC liposomes showed the smallest particle size among all nanoparticles of ~ 50 nm and remained stable after 35 days at 4°C.
- THC showed a much smaller particle size (~400 nm) compared to CBC (~1000) in the emulsion at 4°C over a 35 days period.
- All PDI values were below 0.3, which indicates that there was no aggregation of the particles through 35 days of storage.
- SLNP particles are not as stable as the other two nanoparticles, as variable sizes are seen through the 35 days period.
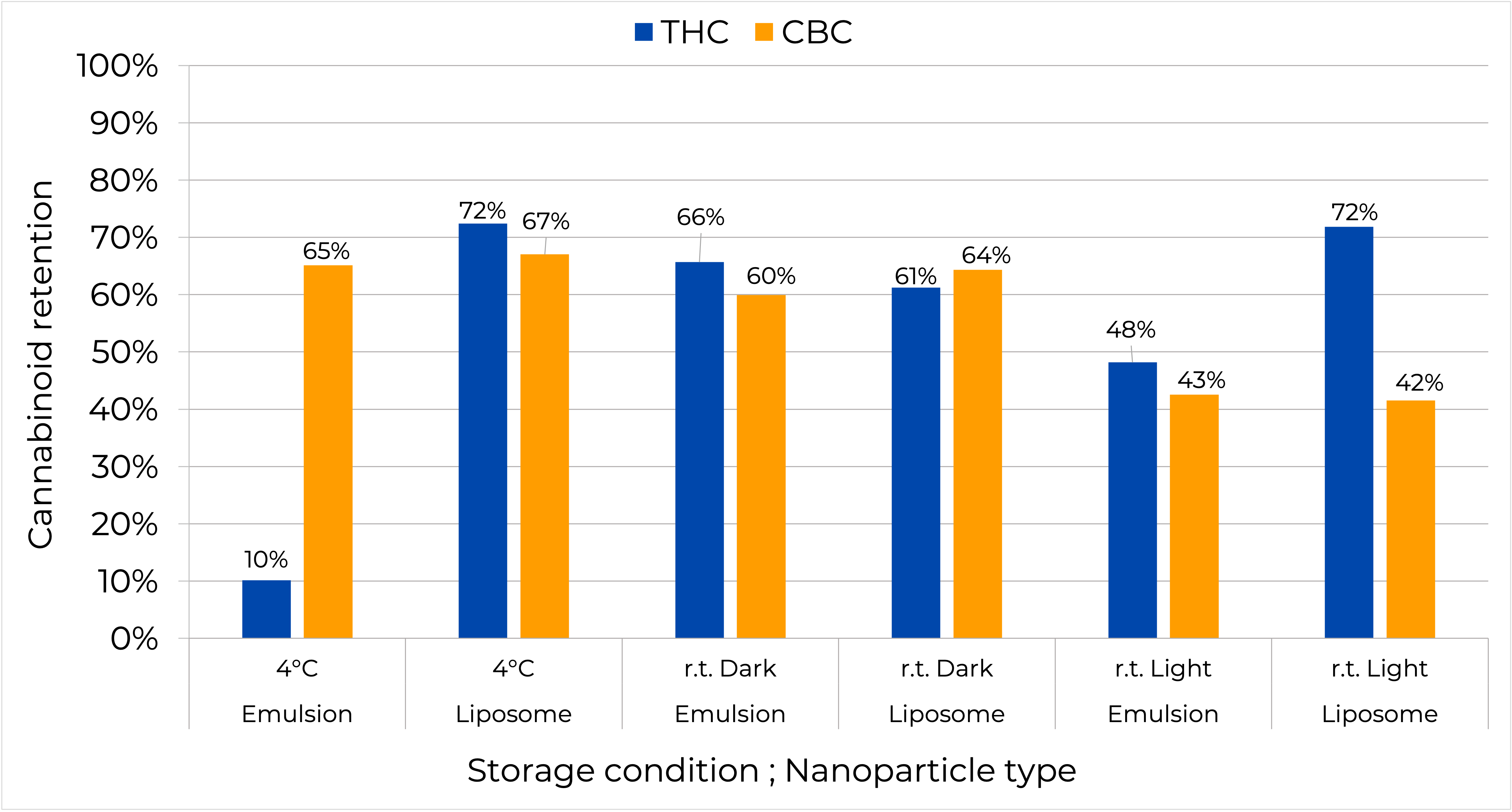
Cannabinoid retention over 35 days of storage at room temperature – dark, light and 4°C conditions.

Figure 5: Cannabinoid retention in the nanoparticle over 35 days of storage at room temperature, dark and light conditions and 4°C
- CBC in both the liposome and emulsion showed higher drug retention at room temperature dark compared to the light condition. The same trend is true for SLNPs, but the difference is not as prominent (data are not shown).
- THC in emulsion showed lower drug retention compared to CBC at 4°C, 10% vs 65% respectively, and similar drug retention between the two cannabinoids in the light and dark conditions.
- In liposomes, both cannabinoids showed similar drug retention when stored at 4°C and r.t. dark. However, when stored at r.t. light condition a lower drug retention for CBC was observed (72% for THC compared to 42% for CBC).
- At the room temperature dark condition, THC and CBC showed similar drug retention in all nanoparticles (around 60%).
CONCLUSION
THC and CBC demonstrate different behaviour in different nanoparticles and conditions. THC and CBC have similar EE% in emulsions and SLNPs. However, EE% between these two cannabinoids are different when formulated into liposomes (lower for CBC). Similar particle size is seen for these two cannabinoids in liposomes at 4°C, while particle size is significantly different in emulsions and SLNPs (larger particle size for CBC in these two nanoparticles). Furthermore, drug retention results show that different types of nanoparticles retain THC and CBC differently in variable conditions. As mentioned before in our previous articles, small differences in the molecular structure of cannabinoids might result in differences in their physicochemical properties and their characterization in nanoparticles. Indeed, more studies are needed to understand the molecules interaction in the interface that causes these changes. CBC has a longer flexible aliphatic side chain compared to THC (Fig. 2), which in the case of liposomes could affect its interaction with lipid tails and placement within the bilayer. EE% values seem to indicate these interactions to be unfavourable for the chosen lipid composition.
Based on these findings, we can conclude that the formulation developed for THC is not optimal for CBC and can be further improved to obtain nanoparticles with desired properties.
FUTURE STUDIES
ASI aims to develop and optimize a formulation that shows a higher EE% (<90%), API stability and bioavailability for CBC based on the formulations explored in this experiment.
Find out more about Ascension Sciences here!
REFERENCES
- Andrea O, Behnoush K, Encapsulating cannabinoids in lipid-based nanoparticles: THC vs. CBD – Part 2 of the series, Pharmaexcipients (2021)
- Malmo-Levine D. Holland J. The Pot Book: A Complete Guide to Cannabis. Rochester, Vermont: Park Street Press (2010)
- Bruni, N. et al. Cannabinoid Delivery Systems for Pain and Inflammation Treatment. Molecules 23, 2478 (2018).
- Das S, Chaudhury A. Recent advances in lipid nanoparticle formulations with solid matrix for oral drug delivery. AAPS PharmSciTech (2011)
- Turner CE, Elsohly MA, Boeren EG . Constituents of Cannabis sativa L. XVII. A review of the natural constituents. J Nat Prod (1980)
- Russo EB . Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br J Pharmacol (2011)
- Howlett AC. et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev (2002)
- Ligresti A. et al. From Phytocannabinoids to Cannabinoid Receptors and Endocannabinoids: Pleiotropic Physiological and Pathological Roles Through Complex Pharmacology. Physiol Rev (2016)
- Izzo, Angelo A, et al. “Inhibitory Effect of Cannabichromene, a Major Non-Psychotropic Cannabinoid Extracted from Cannabis Sativa, on Inflammation-Induced Hypermotility in Mice.” British Journal of Pharmacology, vol. 166, no. 4, (2012)
- De Petrocellis. et al. Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. British Journal of Pharmacology (2011).
- De Petrocellis, L. et al. Cannabinoid actions at TRPV channels: Effects on TRPV3 and TRPV4 and their potential relevance to gastrointestinal inflammation. Acta Physiologica (2012).
- De Petrocellis, L. et al. Plant-derived cannabinoids modulate the activity of transient receptor potential channels of ankyrin type-1 and melastatin type-8. The Journal of Pharmacology and Experimental Therapeutics (2008).
- Shinjyo, N., & Di Marzo, V. The effect of cannabichromene on adult neural stem/ progenitor cells. Neurochemistry International (2013)
- Ligresti A, et al. Antitumor activity of plant cannabinoids with emphasis on the effect of cannabidiol on human breast carcinoma. J Pharmacol Exp Ther (2006).
- TURNER, CARLTON E., and MAHMOUD A. ELSOHLY. “Biological Activity of Cannabichromene, Its Homologs and Isomers.” The Journal of Clinical Pharmacology, (1981)
- DeLong, Gerald T., et al. “Pharmacological Evaluation of the Natural Constituent of Cannabis Sativa, Cannabichromene and Its Modulation by Δ9-Tetrahydrocannabinol☆.” Drug and Alcohol Dependence, vol. 112, no. 1-2, (2010)
- El-Alfy, Abir T., et al. “Antidepressant-like Effect of Δ9-Tetrahydrocannabinol and Other Cannabinoids Isolated from Cannabis Sativa L.” Pharmacology Biochemistry and Behavior, vol. 95, no. 4, (2010)
- Ligresti, Alessia, et al. “Antitumor Activity of Plant Cannabinoids with Emphasis on the Effect of Cannabidiol on Human Breast Carcinoma.” Journal of Pharmacology and Experimental Therapeutics, (2006)
- Oláh, Attila et al. “Differential effectiveness of selected non-psychotropic phytocannabinoids on human sebocyte functions implicates their introduction in dry/seborrhoeic skin and acne treatment.” Experimental dermatology (2016)
- Shinjyo, Noriko, and Vincenzo Di Marzo. “The Effect of Cannabichromene on Adult Neural Stem/Progenitor Cells.” Neurochemistry International, (2013)
- Garrett ER, Hunt CA. Physicochemical properties, solubility, and protein binding of delta9-tetrahydrocannabinol. J Pharm Sci. (1974)
- Pollastro F et al. “Cannabichromene” Nat. Prod. Commun., (2018)

