Studying the API Distribution of Controlled Release Formulations Produced via Continuous Twin-Screw Wet Granulation: Influence of Matrix Former, Filler and Process Parameters
Abstract
Introduction
Controlled release matrix tablets are of high interest to improve patient adherence, minimize fluctuations in drug plasma levels, avoid adverse drug effects and reduce health care costs [1]. Hydroxypropyl methylcellulose (HPMC) is a well-established hydrophilic polymer to formulate controlled release matrix tablets. It is a water-soluble, non-ionic and non-toxic cellulose ether that is enzyme resistant, stable over pH range 3–11 and classified as GRAS by Food and Drug Administration (FDA) [2,3]. Various HPMC grades are available which differ in substitution type (ratio of hydroxypropoxy and methoxy groups) and molecular weight, determining the solubility and gelling rate.
Many studies focused on the processing of HPMC-based controlled release formulations via batch-wise wet granulation techniques, namely high shear and fluidized-bed granulation [4,5,6,7,8,9].
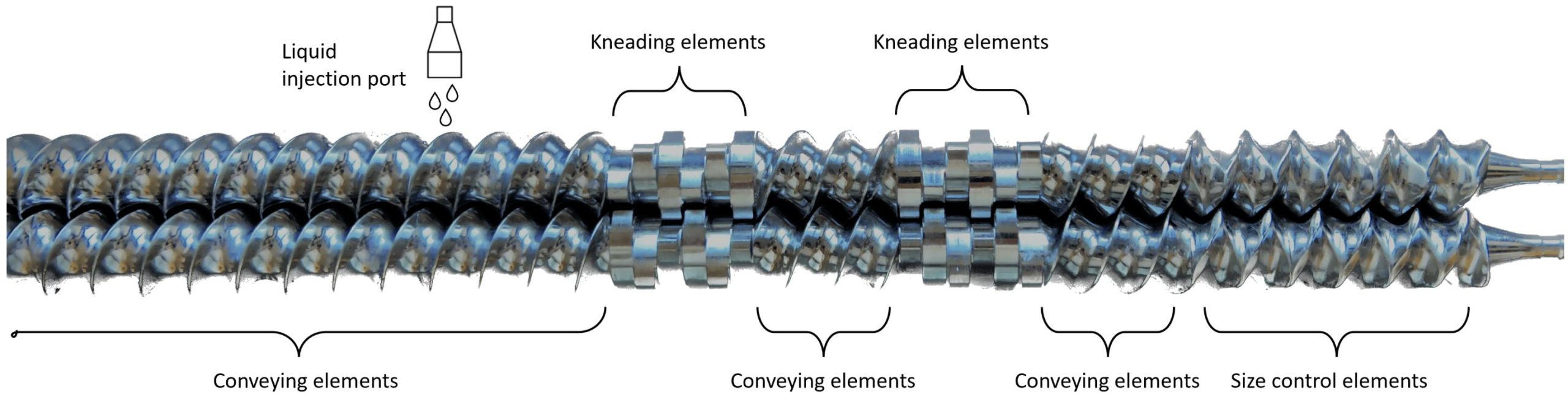
Despite the advantages of continuous manufacturing (improved cost-efficiency, easier scale-up, lower environmental footprint, enhanced flexibility) and the emerging of twin-screw wet granulation (TSWG), the production of HPMC matrix tablets through this continuous technique remains poorly studied [10,11,12,13]. A study of Thompson and O’Donnell [14] processed the controlled release (CR) excipients, HPMC and Kollidon SR, via TSWG. However, this yielded thin noodle-like shape granules due to a ‘rolling’ phenomenon during the granulation process. Altering the screw design was the most effective means to produce near-spherical particles. However, the impact on CR properties of the granules was not studied and no dissolution experiments were performed. Vanhoorne et al. [15] reported the formation of twisted elongated granules only at excessive liquid-to-solid ratio (L/S-ratio) for the same formulation.
However, by excluding microcrystalline cellulose (MCC) as filler from the formulation, the shape of the HPMC granules was similar to those of immediate release formulations. In addition, the influence of filler and process parameters (screw speed, throughput, barrel temperature and screw design) on the granule and tablet properties of an HPMC-based controlled release formulation was studied. In a follow-up study, the influence of the HPMC grade and particle size of theophylline on the critical quality attributes of the granules and tablets was investigated [16]. This showed a non-homogeneous distribution of theophylline over the different sieve fractions with underdosing in the fines fraction (<150 µm), but this could not be correlated to the properties of the HPMC grades.
Vanhoorne et al. [16] reported how to obtain CR granules with similar shape to immediate release formulations, however could not explain the inhomogeneous API distribution over the different sieve fractions. As a homogeneous distribution of the active pharmaceutical ingredient (API) is essential to avoid content uniformity issues during downstream processing (fluidized-bed drying and tableting) and to adhere to the quality-by-design principles, this study investigates the root cause of the non-homogeneous API distribution in CR granules after processing via TSWG. The influence of process (L/S-ratio and screw configuration) and formulation parameters (filler and matrix former type) on the API distribution over the different granule sieve fractions was studied. In addition, the influence of formulation parameters on tableting and dissolution behavior was investigated.
Download the full article as PDF here: Studying the API Distribution of Controlled Release Formulations Produced via Continuous Twin-Screw Wet Granulation
or read it here
Materials
Theophylline was used as slightly soluble model drug (BCS class I) (Siegfried, Zofingen, Switzerland) [17]. Two hydrophilic HPMC matrix formers (90SH-4000SR and 65SH-4000SR) were kindly donated by Shin-Etsu (Niigata, Japan). According to the Ph. Eur., the substitution types of HPMC 90SH and HPMC 65SH are 2208 (19.0–24.0% methoxy and 4.0–12.0% hydroxypropoxy) and 2906 (27.0–30.0% methoxy and 4.0–7.5% hydroxypropoxy) respectively [18]. The hydrophobic matrix former Kollidon® SR was kindly donated by BASF (Ludwigshafen, Germany). Kollidon® SR is a mixture of 80% polyvinyl acetate and 19% povidone (Kollidon® 30). 𝛼-Lactose monohydrate (Pharmatose® 200M, DFE Pharma, Goch, Germany), mannitol (Parteck® M200, Merck, Darmstadt, Germany), dicalcium phosphate (DCP) (Emcompress® Anhydrous Powder, JRS Pharma, Rosenberg, Germany) and MCC (Avicel® PH101, FMC Health and Nutrition, Cork, Ireland) were used as fillers. Pharmatose 200M, Parteck M200 and Emcompress Anhydrous Powder were kindly donated. Magnesium stearate (Ligamed MF-2-V, Peter Greven, Bad Münstereifel, Germany) was used as lubricant during tableting.
Denduyver, P.; Vervaet, C.; Vanhoorne, V. Studying the API Distribution of Controlled Release Formulations Produced via Continuous Twin-Screw Wet Granulation: Influence of Matrix Former, Filler and Process Parameters. Pharmaceutics 2024, 16, 341. https://doi.org/10.3390/pharmaceutics16030341
Read also our introduction article on Mannitol here:


