De novo development of small cyclic peptides that are orally bioavailable
Abstract
Cyclic peptides can bind challenging disease targets with high affinity and specificity, offering enormous opportunities for addressing unmet medical needs. However, as with biological drugs, most cyclic peptides cannot be applied orally because they are rapidly digested and/or display low absorption in the gastrointestinal tract, hampering their development as therapeutics. In this study, we developed a combinatorial synthesis and screening approach based on sequential cyclization and one-pot peptide acylation and screening, with the possibility of simultaneously interrogating activity and permeability. In a proof of concept, we synthesized a library of 8,448 cyclic peptides and screened them against the disease target thrombin. Our workflow allowed multiple iterative cycles of library synthesis and yielded cyclic peptides with nanomolar affinities, high stabilities and an oral bioavailability (%F) as high as 18% in rats. This method for generating orally available peptides is general and provides a promising push toward unlocking the full potential of peptides as therapeutics.
Main
One of the biggest bottlenecks in therapeutic development is that a large proportion of the proteins can currently not be targeted by classical small molecules administered orally1. Challenging targets include proteins with flat, featureless surfaces to which small molecules can hardly bind. Even targets such as enzymes, receptors or ion channels, which have pockets or clefts for ligand binding, can occasionally be challenging, for example, when selectivity for a specific protein homolog is required. The much larger biologics that can selectively target these interactions require injection2. A solution could potentially be offered by cyclic peptides due to their ability to bind challenging targets with high affinity while being relatively small and, thus, in reach of being orally available3,4. In fact, a small number of peptides derived from natural sources developed into oral drugs indicate the feasibility of orally available peptides, including cyclosporine A, desmopressin and a somatostatin analogue5,6. However, the vast majority of natural and de novo developed peptides have a large size and polar surface, or are proteolytically not stable, which forces administration by injection. If it was possible to develop target-selective peptides that are orally available, several unmet medical needs could be addressed7,8
Toward the generation of oral peptides to new targets, an in-depth comparison of physicochemical parameters from orally available versus non-available examples pointed to the molecular weight (MW), the number of hydrogen bond donors (HBDs) and the polar surface area (PSA) as the most decisive factors9,10. Most oral peptides have a molecular weight below 700 Da, a polar surface smaller than 200 Å2 and five or fewer H-bond donors5,11. This is not a hard rule, though, as shown by the much larger cyclosporine A (CsA; 1,203 Da) that has many N-methylated amide bonds and can adapt a conformation to limit its polar surface. Studies with model peptides showed that N-methylation can be applied strategically to reach an oral availability as high as 30% for cyclic hexapeptides12,13,14. Most recently, computationally designed cyclic peptides with as many as 12 amino acids, with amide groups engaging in internal hydrogen bonding interactions for shielding the polar surface, were shown to permeate cellular membranes and be orally available in murine models15. However, as the strategically N-methylated model peptides, these peptides still need to be furnished with target-binding properties for drug development. Taken together, there are increasing data showing that cyclic peptides can be orally available, but the de novo generation of target-specific, orally available peptides still poses a formidable challenge.
For generating cyclic peptides that are target specific and have the required physicochemical boundaries, large libraries were screened by phage or mRNA display16,17. For instance, we recently applied phage display to generate cyclic peptides to withstand the proteolytic pressure of the gastrointestinal tract, but even our smallest peptides were still too large (9-mer; 1,134 Da), were too polar and had almost no oral bioavailability in mice (0.2%)18. Overall, biological display libraries appear to have an insufficient chemical and structural diversity to develop short peptides (for example, 6-mers) that have a high affinity, especially if the libraries are limited to mostly canonical amino acids. DNA-encoded libraries (DELs) allow a much wider choice of hundreds of unnatural amino acids and other chemical building blocks and were used for screening cyclic peptide and macrocycle libraries19. Although the synthesis of cyclic peptide DELs is challenging due to the large number of sequential coupling and deprotection steps in the presence of DNA, promising results have been achieved in recent years20,21. With the goal of generating chemically and structurally highly diverse libraries that can be screened by functional assays instead of affinity selections, we developed strategies in which cyclic peptides are combinatorially synthesized in microwell plates at a nanomole scale via acoustic dispensing and can be rapidly screened as crude products in the same plates using diverse types of assays22,23,24. For example, we generated 20,000 cyclic peptides by diversifying around 200 disulfide-cyclized peptides carrying an amino group with 100 carboxylic acids and identified nanomolar binders24. However, the labile disulfide bridge in these peptides rendered them unsuitable for oral application, but no other suitable cyclization chemistries were readily compatible with this rapid, one-pot synthesis and screening technology.
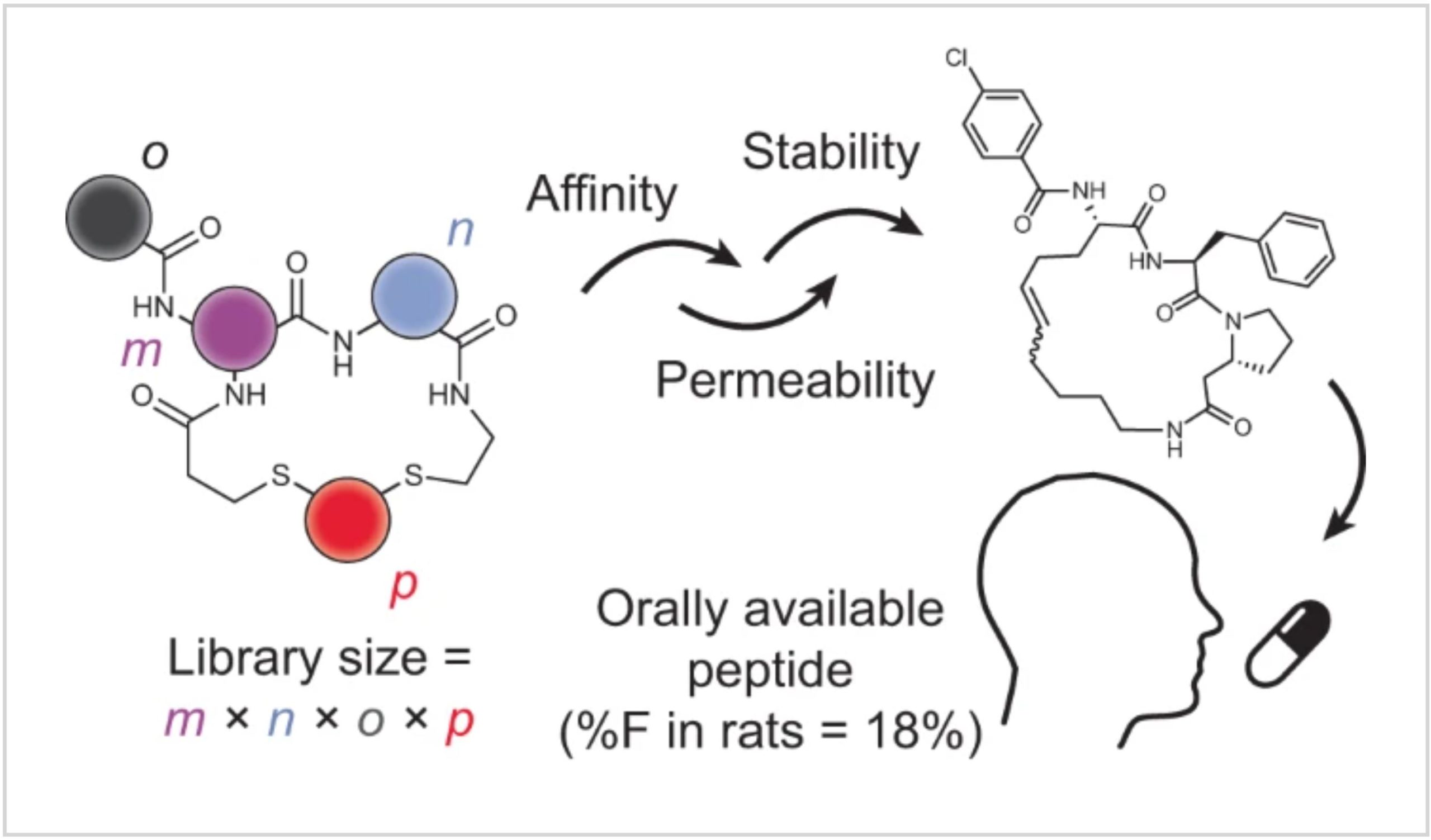
In this work, we expanded the above-described nanomole-scale peptide library synthesis to function with thioether-cyclized peptides that are more metabolically stable upon oral administration than the previously used disulfide-cyclized peptides. For synthesizing the libraries, we conceived a two-step combinatorial synthesis strategy in which ‘m’ random peptides are cyclized by ‘n’ linkers to obtain thioether-cyclized peptides, which are then acylated at a peripheral amine with ‘o’ carboxylic acids to yield m × n × o cyclic peptides (Fig. 1a). We first assessed if this peptide format is metabolically stable and then established conditions and procedures to combine the two combinatorial diversification steps. We used this approach to synthesize and screen a large library against the disease target thrombin25. Finally, we modified the library screening approach to include readouts for stability and membrane permeability, which we used to iteratively improve these qualities in our hit peptides. In the end, this work culminated in a five-building-block cyclic peptide that displayed an oral availability of 18% in rats. Overall, this work provides a set of synthesis and screening tools and a workflow that can be implemented for any target interaction that has a functional readout to move the field closer to the longstanding goal of developing target-specific peptides that can be administrated orally.
Download the full article as PDF here De novo development of small cyclic peptides that are orally bioavailable
or read it here
Merz, M.L., Habeshian, S., Li, B. et al. De novo development of small cyclic peptides that are orally bioavailable. Nat Chem Biol (2023). https://doi.org/10.1038/s41589-023-01496-y
Read more on Orally Disintegrating Tablets (ODTs) here:


