Innovative Design of Targeted Nanoparticles: Polymer–Drug Conjugates for Enhanced Cancer Therapy
Abstract
Polymer–drug conjugates (PDCs) have shown great promise in enhancing the efficacy and safety of cancer therapy. These conjugates combine the advantageous properties of both polymers and drugs, leading to improved pharmacokinetics, controlled drug release, and targeted delivery to tumor tissues. This review provides a comprehensive overview of recent developments in PDCs for cancer therapy. First, various types of polymers used in these conjugates are discussed, including synthetic polymers, such as poly(↋-caprolactone) (PCL), D-α-tocopheryl polyethylene glycol (TPGS), and polyethylene glycol (PEG), as well as natural polymers such as hyaluronic acid (HA). The choice of polymer is crucial to achieving desired properties, such as stability, biocompatibility, and controlled drug release. Subsequently, the strategies for conjugating drugs to polymers are explored, including covalent bonding, which enables a stable linkage between the polymer and the drug, ensuring controlled release and minimizing premature drug release.
The use of polymers can extend the circulation time of the drug, facilitating enhanced accumulation within tumor tissues through the enhanced permeability and retention (EPR) effect. This, in turn, results in improved drug efficacy and reduced systemic toxicity. Moreover, the importance of tumor-targeting ligands in PDCs is highlighted. Various ligands, such as antibodies, peptides, aptamers, folic acid, herceptin, and HA, can be incorporated into conjugates to selectively deliver the drug to tumor cells, reducing off-target effects and improving therapeutic outcomes. In conclusion, PDCs have emerged as a versatile and effective approach to cancer therapy. Their ability to combine the advantages of polymers and drugs offers enhanced drug delivery, controlled release, and targeted treatment, thereby improving the overall efficacy and safety of cancer therapies. Further research and development in this field has great potential to advance personalized cancer treatment options.
Introduction
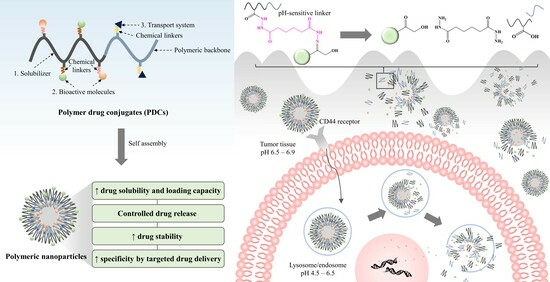
Cancer treatment poses a significant challenge to medicinal sciences. Although chemotherapy and radiation therapy are primary therapeutic strategies, they often cause severe systemic side effects [1]. In addition, the low solubility of many chemotherapeutics causes aggregation, triggering an immune response and clearance from the body. This ultimately decreases the circulation time in the bloodstream and reduces its effectiveness in delivering the free drug to tumor sites [2,3]. Polymer–drug conjugates (PDCs) are drug delivery technologies that were first initiated by Horst Jatzkewitz in 1955 [4]. Several drug molecules are covalently bound to polymeric carriers through bioresponsive linkers to improve stability with the diversity, specificity, and functionality of biomolecules [5]. PDCs offer various advantages for cancer therapy. They can improve drug solubility and loading capacity [6,7], improve pharmacokinetic profiles by controlling and maintaining drug release [8,9], and increase drug half-life by decreasing immune system recognition. In addition, they increase drug accumulation specificity at the target site through passive and active transport [10,11,12,13].
The design and synthesizing of new PDCs that can interact effectively with biological systems is a challenge. Drugs must have free functional groups that can be conjugated directly to polymer backbones through chemical linkers (Figure 1); otherwise, PDCs are impossible to form. For example, curcumin (CUR) presents the functional group of R-OH and R-C=O-R, as seen in Figure 1. These functional groups can be linked to R-C=O-OH and R-HN2 of the polymer to form ester and hydrazone linkers, respectively. PDCs also enable the codelivery of drugs and/or bioactive molecules with different properties in one nanoparticle, making them multifunctional [14,15]. Due to these advantages over the free form of a drug, PDCs have been widely applied in medicinal treatments for various diseases such as cancer, osteoporosis, infection, and immunodeficiency. The focus of this article is to review the rational design of PDCs for cancer therapy. PDCs of various chemistries and architectures have been discussed, with particular emphasis on ideas for enhancing PDC systems.
Chapter 4.1.2 with the focus on TPGS – D-α-tocopheryl polyethylene glycol:
4.1.2. TPGS
TPGS is a water-soluble derivative of natural vitamin E, which is formed via esterification with PEG (MW of 1000). The structure is shown in Figure 6. It is composed of hydrophobic and hydrophilic segments in its structure, which present amphiphilic properties. Therefore, it has been widely used in pharmaceutically safe adjuvants as a wetting agent, emulsifier, stabilizer, and solubilizing agent [54]. Recently, TPGS has become more attractive in the field of drug delivery systems as a nanocarrier because it can improve the solubility and bioavailability of poorly water-soluble and poorly absorbed drugs [55,56,57,58]. Its safety has been reported, with the oral 50% lethal dose (LD50) being >7 g/kg for young adult rats of both sexes [59]. In addition, the US FDA has approved TPGS as a safe and biocompatible adjuvant. Most reports showed that TPGS has been prepared in prodrugs, in which TPGS is conjugated with drugs to improve the pharmacokinetic profile of drug molecules. Mi et al. reported the synthesis of the TPGS-cisplatin (CIS) conjugate, which exhibited pH-dependent drug release, much higher cellular uptake, and higher cellular cytotoxicity compared to the unconjugated drug [60]. Our previous work also developed targeted PDCs using folic-acid-conjugated TPGS to deliver methotrexate (MTX). The results showed that these copolymers potentiated cytotoxicity and cellular uptake efficiency for breast cancer cells [61].
Some publications conjugated TPGS with other polymers to improve their properties. For example, TPGS-b-PCL copolymers have been utilized for drug delivery, with the aim of achieving the combined benefits of TPGS and PCL to increase the hydrophobicity of the copolymer and help with water-insoluble drugs. These copolymers have shown successful applications in cancer therapy by increasing drug loading and cytotoxic activity in liver cancer [62]. Another example is chitosan conjugated with TPGS and further decorated with transferrin, which was used to form targeted nanocarriers to deliver docetaxel (DTX). It provided a bioadhesive property and cytotoxicity that were useful for brain cancer therapy [63]. Nowadays, TPGS is widely investigated to overcome multidrug resistance (MDR) because TPGS has shown inhibitory activity to P-glycoprotein (P-gp) and potent antitumor activity, resulting in enhanced bioavailability of drugs such as MTX, DTX, DOX, CIS, and PTX [64,65,66]. Almawash et al. [67] successfully boosted the cytotoxicity of MTX by using PLGA-TPGS. The results revealed that the conjugation of MTX-PLGA-TPGS provided an improved pharmacokinetic profile and increased drug stability in the blood circulation as a result of the properties of TPGS. This led to increased cellular uptake and improved drug efficiency for cancer treatment, such as antibodies [68], galactosamine [69], and folic acid [61].
In addition, some targeting ligands can be decorated on TPGS to enhance cellular uptake. Gan et al. [68] investigated novel sorafenib (Sf)-loaded polymeric nanoparticles for the targeted therapy of hepatocellular carcinoma. Anti-GPC3 antibody (Ab) and Sf were grafted onto a TPGS-PLC block copolymer, which was further self-assembled from nanoparticles. The result showed that NP-Sf-Ab showed robust stability and achieved excellent Sf release in the cell medium. The MTT assay confirmed that NP-Sf-Ab caused much higher cytotoxicity than non-targeted NP-Sf and free Sf. Finally, NP-Sf-Ab was shown to greatly inhibit tumor growth in HepG2-xenograft-bearing nude mice without obvious side effects. Examples of useful TPGS based on PDCs for cancer drug delivery are listed in Table 2.
Download the full article as PDF here Innovative Design of Targeted Nanoparticles: Polymer–Drug Conjugates for Enhanced Cancer Therapy
or read it here
Junyaprasert, V.B.; Thummarati, P. Innovative Design of Targeted Nanoparticles: Polymer–Drug Conjugates for Enhanced Cancer Therapy. Pharmaceutics 2023, 15, 2216. https://doi.org/10.3390/pharmaceutics15092216

