Development of a Discriminative Dissolution Method, Using In-Silico Tool for Hydrochlorothiazide and Valsartan Tablets
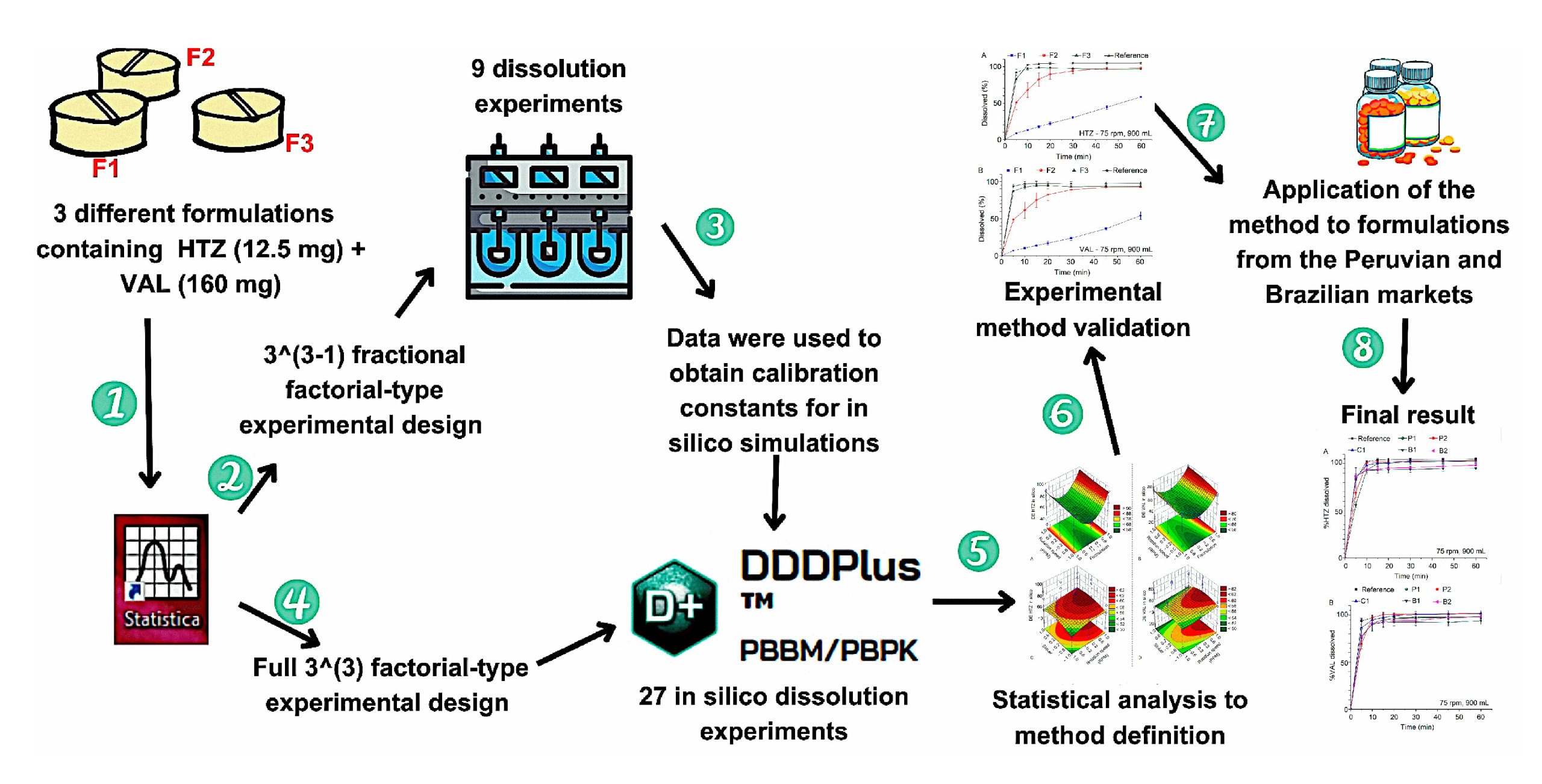
Hydrochlorothiazide (HTZ) and Valsartan (VAL) are poorly soluble drugs in BCS classes IV and II. This study aimed to develop a method to assess the dissolution profile of tablets containing HTZ (12.5 mg) and VAL (160 mg) as a fixed-dose combination, using in silico tools to evaluate products marketed in Brazil and Peru. Firstly, in vitro dissolution tests were performed using a fractional factorial design 33−1. Then, DDDPlus™ was used to carry out experimental design assays of a complete factorial design 33. Data from the first stage were used to obtain calibration constants for in silico simulations. The factors used in both designs were formulation, sinker use, and rotation speed. Finally, effects and factor interaction assessment was evaluated based on a statistical analysis of the dissolution efficiency (DE) obtained from simulations. Thus, the established final conditions of the dissolution method were 900 mL of phosphate buffer pH 6.8, 75 rpm of rotation speed, and sinker use to prevent formulation floating. The reference product stood out because of its higher DE than other formulations. It was concluded that the proposed method, in addition to ensuring total HTZ and VAL release from formulations, has adequate discriminative power.
2.1. Materials
Download the full study as PDF here: Development of a Discriminative Dissolution Method, Using In-Silico Tool for Hydrochlorothiazide and Valsartan Tablets
or read it here
Leon, R.M.; Issa, M.G.; Duque, M.D.; Daniel, J.S.P.; Ferraz, H.G. Development of a Discriminative Dissolution Method, Using In-Silico Tool for Hydrochlorothiazide and Valsartan Tablets. Pharmaceutics 2023, 15, 1735.
https://doi.org/10.3390/pharmaceutics15061735

