Cleaning of Direct Compression Continuous Manufacturing Equipment through displacement of API residues by excipients
Abstract
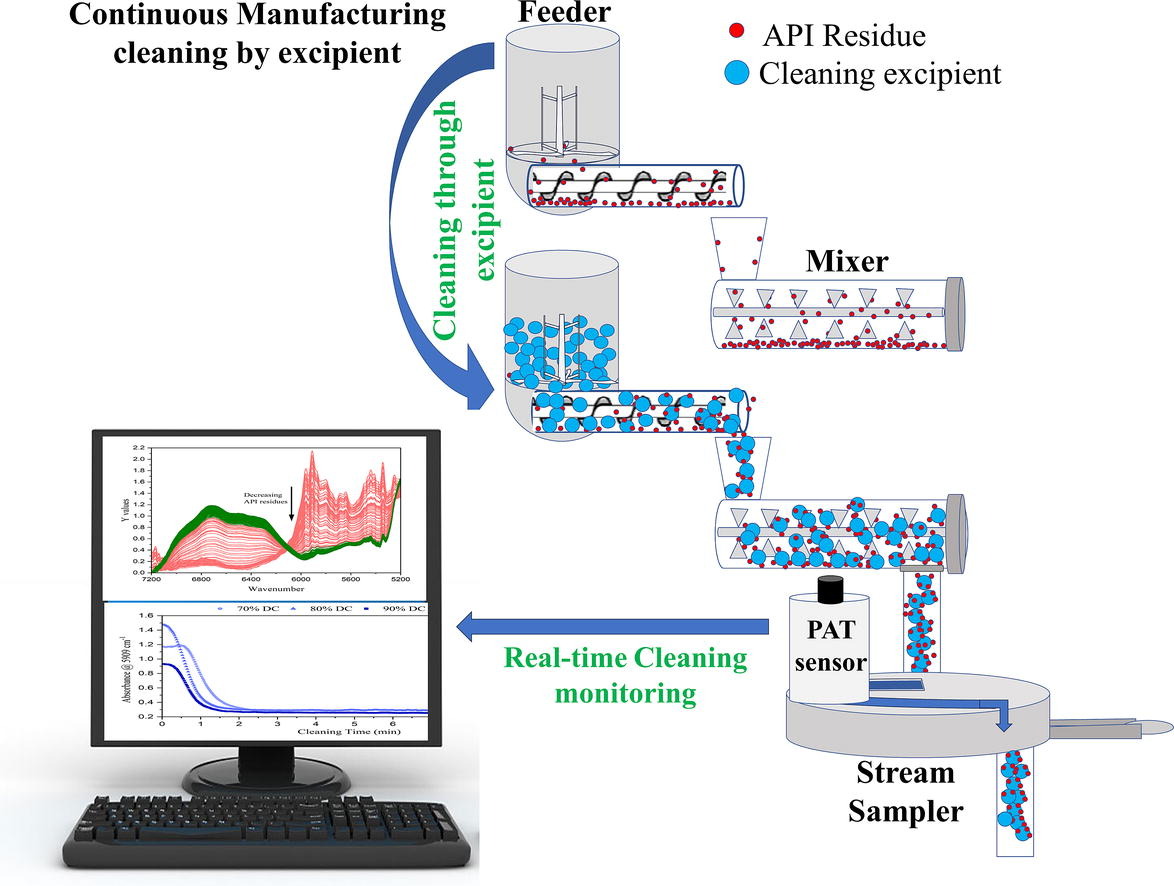
This feasibility study evaluates a cleaning process designed to avoid the use of detergents and reduce operator exposure to the active pharmaceutical ingredient (API). The continuous manufacturing equipment was cleaned using excipients to displace ibuprofen residues from the system. The cleaning process was performed using 3.0 kg of Prosolv® and 3.0 kg of Tablettose® 70. The impact of different volumetric feed rates of the cleaning excipient was assessed. The displacement of API and blend residues was evaluated with in-line near infrared (NIR) spectroscopy. Principal component analysis (PCA) was performed to evaluate the cleaning progress as the Prosolv® flowed through the feeder, mixer and stream sampler. In-place Raman spectra were acquired from the material sticking to detect the ibuprofen residues. The study showed that Prosolv® and Tablettose® can remove ibuprofen residues effectively from the hopper, feeder screw, mixer paddles, shaft and stream sampler. The Process Analytical Technology (PAT) system can be utilized to detect API displacement during the cleaning process. However, dismantling and manual cleaning was required to remove material sticking at the surfaces adjacent to the rotating feeder screws and mixer paddles.
Introduction
The cleaning of pharmaceutical processing equipment is challenging and time-intensive and often uses organic solvents, detergents, heating and/or extensive cleaning cycles (Lopolito and Rivera, 2017, Markarian, 2021, Peles et al., 2013). The risk assessment of these critical cleaning parameters is another challenge (Lopolito and Rivera, 2016). Cleaning procedures are expected to meet the optimum changeover time as established in the production schedule. Frequent cleaning may be required to remove material buildup during a campaign of multiple batches or lots (Konge and Subramanian, 2022, Stefansdottir et al., 2017). The buildup of material adhered to surfaces could alter the critical quality attributes (Allenspach et al., 2021, Bekaert et al., 2022b, Bekaert et al., 2022c, Bekaert et al., 2022a, Engisch and Muzzio, 2016).
Pharmaceutical companies with continuous manufacturing (CM) processes have adopted a lean manufacturing approach with dedicated operators and separate cleaning facilities for efficient and faster cleaning (Markarian, 2021). They have also organized the parts for a continuous rig and unidirectional movement (Conway et al., 2023). As an example, all feeder parts can be placed together to prevent the mix-up of the components. A recent article indicated that Chinoin pharmaceutical’s CM system used to take approximately three shifts to clean the line. However, by being much more familiar with the equipment, they can reduce product changeovers to 12 hours (Manufacturing Chemist, 2023). Previous studies have also improved the analytical methods required for cleaning validation (Köhler et al., 2015, Korčok and Tršić-Milanović, 2017, Lambropoulos et al., 2000, Li et al., 2018). Nevertheless, the discussion of how to facilitate the cleaning of CM has been limited in spite of the significant progress made (Conway et al., 2023, Holman et al., 2021, Rosas et al., 2023).
A clean-in-place or automated cleaning procedure for CM systems would be advantageous over manual cleaning. The integrated PAT sensors in the CM system could aid in the cleaning process evaluation. A cleaning procedure is developed with the excipients used in the formulation to remove API residues from the CM system. In this way, when the equipment is dismantled in a final step the operator encounters mostly excipients instead of API and avoiding risking their health. The proposed approach could also reduce the time needed to clean the CM equipment by facilitating the removal of API residues and reducing the number of parts that need to be dismantled.
The results section of this study is divided according to the two objectives of the study. Section 3.1 describes the first efforts to develop a clean-in-place protocol to reduce the downtime for product and process changeover. In this study, the word cleaning refers to the use of flowing formulation excipients to clean the CM system by removing ibuprofen residues. Cleaning was monitored using the same near-infrared spectrometer and interface that is used in monitoring blending. Section 3.2 describes the cleaning and visual inspection of the CM system. This study describes the first efforts to make the cleaning process easy and less cumbersome without the use of detergents and reducing the dismantling of the CM system.
Read more here
Materials
Ibuprofen DC 85 W (Ph.Eur./USP/JP/IP, BASF Corporation/Bishop, Texas, USA) was selected as a representative granular active pharmaceutical ingredient (API). The excipients used were silicified microcrystalline cellulose (Prosolv® SMCC HD90, JRS Pharma GMBH& Co.), lactose monohydrate (Tablettose® 100 and Tablettose® 70, Agglomerated, Ph.Eur., USP/NF/JP, Molkerei MEGGLE Wasserburg GMBH & Co.), and magnesium stearate
Dhavalkumar S. Patel, Rafael Méndez, Rodolfo J Romañach, Cleaning of Direct Compression Continuous Manufacturing Equipment through displacement of API residues by excipients, International Journal of Pharmaceutics, 2024, 123849, ISSN 0378-5173, https://doi.org/10.1016/j.ijpharm.2024.123849.
Read more on Magnesium Stearate here:


