Quantitative imaging of doxorubicin diffusion and cellular uptake in biomimetic gels with human liver tumor cells
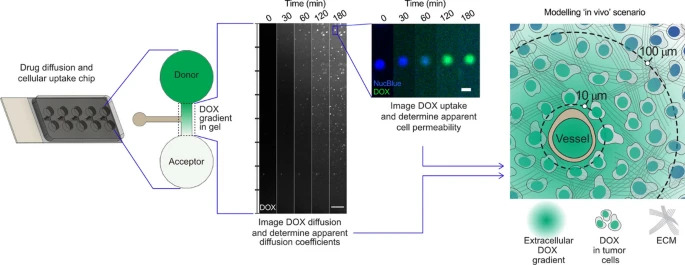
Novel tumor-on-a-chip approaches are increasingly used to investigate tumor progression and potential treatment options. To improve the effect of any cancer treatment it is important to have an in depth understanding of drug diffusion, penetration through the tumor extracellular matrix and cellular uptake. In this study, we have developed a miniaturized chip where drug diffusion and cellular uptake in different hydrogel environments can be quantified at high resolution using live imaging. Diffusion of doxorubicin was reduced in a biomimetic hydrogel mimicking tissue properties of cirrhotic liver and early stage hepatocellular carcinoma (373 ± 108 µm2/s) as compared to an agarose gel (501 ± 77 µm2/s, p = 0.019). The diffusion was further lowered to 256 ± 30 µm2/s (p = 0.028) by preparing the biomimetic gel in cell media instead of phosphate buffered saline. The addition of liver tumor cells (Huh7 or HepG2) to the gel, at two different densities, did not significantly influence drug diffusion. Clinically relevant and quantifiable doxorubicin concentration gradients (1–20 µM) were established in the chip within one hour. Intracellular increases in doxorubicin fluorescence correlated with decreasing fluorescence of the DNA-binding stain Hoechst 33342 and based on the quantified intracellular uptake of doxorubicin an apparent cell permeability (9.00 ± 0.74 × 10–4 µm/s for HepG2) was determined. Finally, the data derived from the in vitro model were applied to a spatio-temporal tissue concentration model to evaluate the potential clinical impact of a cirrhotic extracellular matrix on doxorubicin diffusion and tumor cell uptake.
Introduction
In vitro 2D and 3D cell models form the backbone of pre-clinical cancer research. Combining such models with microfluidic solutions, which are typically compatible with high resolution imaging techniques, permit more complexity to be studied in vitro and enables tumor-on-a-chip approaches which may be more clinically relevant within drug development [1, 2]. In such devices gradients of nutrients, oxygen and cellular waste products can be formed as a result of cell metabolism, pressure differences, convective transport and diffusion similar to that seen in vivo [3,4,5]. However, challenges in terms of drug adsorption to materials as well as physiological relevance of the applied volumes, ratios and flows remain [1, 2].
Therefore, simpler miniaturized systems without fluidics may be applied to create in vitro models in which concentration gradients of drugs can be established in various disease-relevant matrices, thereby focusing on crucial in vivo processes such as drug diffusion, cellular uptake, tissue exposure and drug action [5,6,7]. These properties are important to establish, as it will assist the development of drug molecules that effectively diffuse across the tumor extracellular matrix (ECM) to reach intracellular targets and erase all cancer cells, which is crucial to prevent cancer resistance and recurrence [6, 8]. Additionally, such properties may also be implemented in various in silico modelling approaches in order to translate in vitro findings to a wider clinical context [2, 9, 10].
Hydrogels containing tumor cells are often used for modelling tumor tissue in vitro and low melting point (65.5 °C) agarose is especially useful for the development of such novel miniaturized systems as it provides ease of preparation in aqueous media and gels at room temperature. Investigating diffusion in such gels may provide an accurate estimation of unhindered drug diffusion, i.e. free diffusion in water [10,11,12]. This in turn means that the Stokes-Einstein equation can be applied to predict, with good approximation, diffusion coefficients in such agarose hydrogels.
Hepatocellular carcinoma (HCC) is a primary liver tumor that usually develops on a background of chronic liver disease and progresses from fibrosis to cirrhosis [13]. These changes are characterized by the extensive deposition of the ECM proteins collagen and fibrin, which change the biomechanical properties of the liver and increase its stiffness as the disease progresses. This progression can be mimicked in vitro by the development of biomimetic hydrogels containing collagen and fibrin [14]. The gels stiffness can be tuned to biophysically resemble both healthy and diseased mice liver tissue [15], and the diffusion coefficients of piroxicam and human lactoferrin, which encompass a molecular mass (M) range from 331 to 79 000 g/mol, are significantly reduced in the biomimetic gels compared with diffusion in agarose gels [10].
Doxorubicin (DOX; M = 543.5 g/mol) is a clinically relevant and commonly investigated anticancer drug with two different mechanisms of action, which both activate apoptotic pathways. Firstly, DOX can intercalate between DNA base pairs to prevent DNA replication. The second mechanism of action is the intracellular generation of reactive oxygen species [16,17,18]. Severe side-effects associated with DOX treatment have been reported, such as cardiotoxicity, bone marrow toxicity and intestinal mucositis [19,20,21]. Therefore, locoregional treatments with DOX such as emulsion-based transarterial chemoembolization (TACE) [22], or systemic treatments using nanoparticles such as liposomal formulations [23], are attractive in the clinical setting as these strategies offer targeted drug accumulation at the tumor site as well as reduced side-effects.
The molecular structure of DOX allows for relatively simple quantification of drug concentration via both absorbance- and fluorescence-based techniques [24]. In biological in vitro settings fluorescence-based techniques have proven more useful since many hydrogel and cell culture components contribute challenging background absorbance in the UV spectrum [10]. Quantifying DOX via fluorescence must however be performed with caution since the fluorescence emission spectra for DOX overlaps with the emission spectra for some commonly used stains in fluorescence microscopy (e.g. the cell death reporter propidium iodide). Additionally, the fluorescence signal of DOX can be both enhanced or quenched by lipids and other cellular components [25, 26]. One solution to this challenge, initially proposed by Hovorka et al. [27], is to study the signal from Hoechst stains (e.g. H33342) as an indirect readout for DOX concentration, as the Hoechst molecule competes with DOX for DNA binding. Therefore, a decrease in the observed Hoechst signal correlates with an increase in the nuclear concentration of DOX and permits indirect assessment of intracellular DOX concentrations.
The primary objective of this study was to develop a miniaturized chip for fluorescence-based visualization and quantification of DOX diffusion in biomimetic hydrogels that mimic tissue properties of cirrhotic liver and early stage HCC. Secondly, human liver tumor cells were added to the biomimetic gels and the influence of cells on DOX diffusion, as well as intracellular DOX uptake, was investigated. Finally, the implications of our in vitro findings were translated to a clinical scenario using spatio-temporal tissue concentration modelling.
Download the full study as PDF here: Quantitative imaging of doxorubicin diffusion and cellular uptake in biomimetic gels with human liver tumor cells
or read it here
Degerstedt, O., O’Callaghan, P., Clavero, A.L. et al. Quantitative imaging of doxorubicin diffusion and cellular uptake in biomimetic gels with human liver tumor cells. Drug Deliv. and Transl. Res. (2023).
https://doi.org/10.1007/s13346-023-01445-1

