Enteric coating of tablets containing an amorphous solid dispersion of an enteric polymer and a weakly basic drug: a strategy to enhance in vitro release
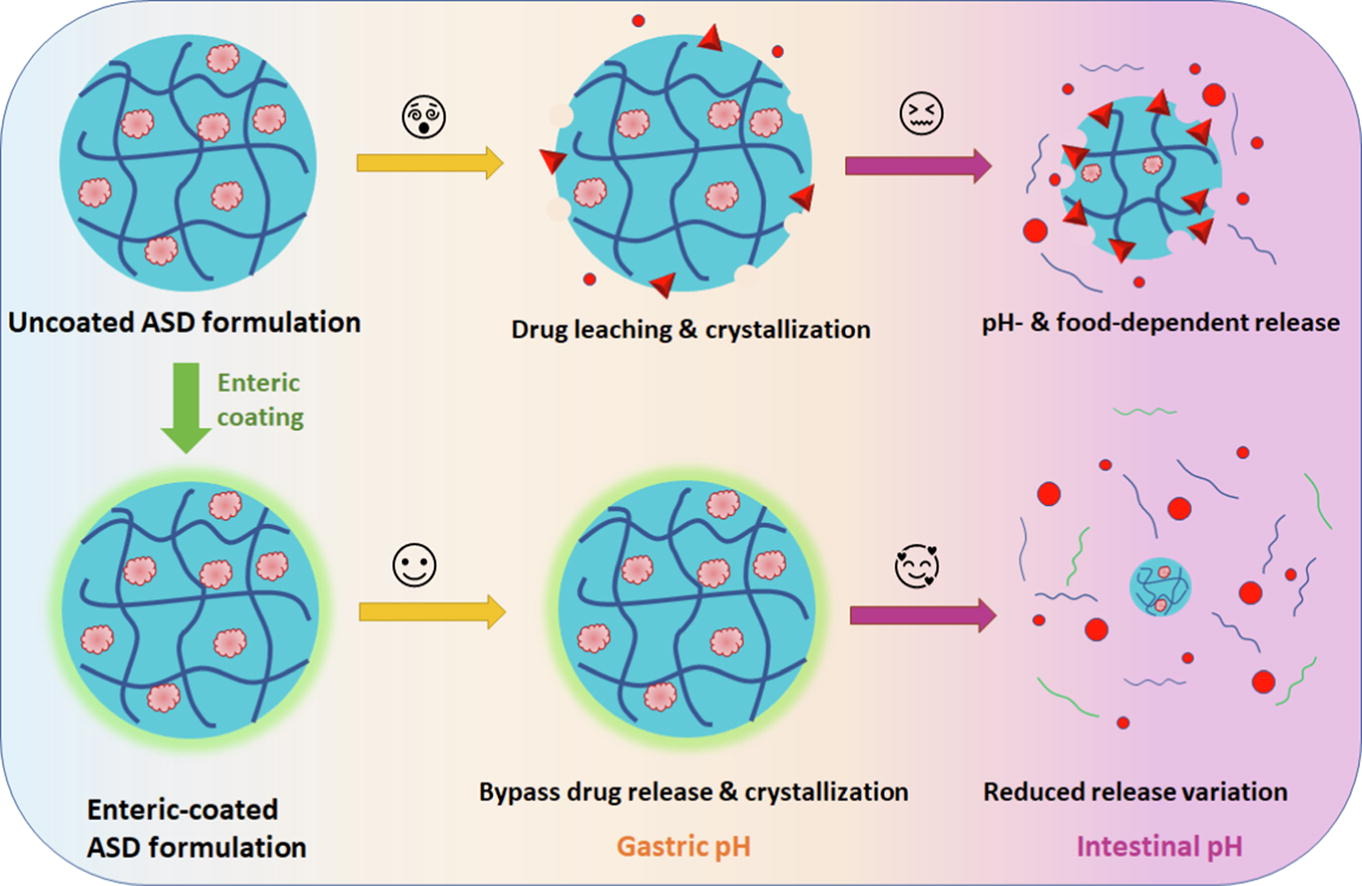
Recent work has highlighted that amorphous solid dispersions (ASDs) containing delamanid (DLM) and an enteric polymer, hypromellose phthalate (HPMCP), appear to be susceptible to crystallization during immersion in simulated gastric fluids. The goal of this study was to minimize contact of the ASD particles with the acidic media via application of an enteric coating to tablets containing the ASD intermediate, and improve the subsequent drug release at higher pH conditions. DLM ASDs were prepared with HPMCP and formulated into a tablet that was then coated with a methacrylic acid copolymer (Acryl EZE II®). Drug release was studied in vitro using a two-stage dissolution test where the pH of the gastric compartment was altered to reflect physiological variations. The medium was subsequently switched to simulated intestinal fluid. The gastric resistance time of the enteric coating was probed over the pH range of 1.6-5.0.
Highlights
- Variation of release from delamanid ASDs with an enteric polymer in different physiologically relevant conditions was due to the high crystallization tendency of the drug.
- Enteric coating protected against drug crystallization from ASDs in simulated gastric fluids.
- Enteric coating reduced the impact of pH and food components on in vitro drug release from dosage forms containing an ASD of delamanid.
The enteric coating was found to be effective at protecting the drug against crystallization in pH conditions where HPMCP was insoluble. Consequently, the variability in drug release following gastric immersion under pH conditions reflecting different prandial states was notably reduced when compared to the reference product. These findings support closer examination of the potential for drug crystallization from ASDs in the gastric environment where acid-insoluble polymers may be less effective as crystallization inhibitors. Further, addition of a protective enteric coating appears to provide a promising remediation strategy to prevent crystallization at low pH environments, and may mitigate variability associated with prandial state that arises due to pH changes.
Download the full article as PDF here Enteric coating of tablets containing an amorphous solid dispersion of an enteric polymer and a weakly basic drug: a strategy to enhance in vitro release
or read it here
Materials
Delamanid (DLM) was obtained from Gojira Fine Chemicals, LLC (Bedford Heights, OH) while Deltyba® tablets were manufactured by Otsuka Pharmaceutical Co., Ltd., (Tokyo, Japan). Hydroxypropyl methylcellulose phthalate (HPMCP, P-50 grade) was from Shin-Etsu Chemical Co., Ltd. (Tokyo, Japan). 1,2-Ethanedisulfonic acid dihydrate was supplied by Tokyo Chemical Industry Co. Ltd. (Tokyo, Japan). Croscarmellose sodium (Ac-Di-Sol®) and microcrystalline cellulose PH 101 were sourced from FMC Biopolymer (Newark, DE). Sodium starch glycolate (SSG) was purchased from JRS Pharma (Posenberg, Germany). Silica, colloidal anhydrous (Aerosil® 200) and Eudragit® L100-55 were provided by Evonik (Darmstadt, Germany). Magnesium stearate was procured from Spectrum (New Brunswick, NJ). Acryl-EZE® II was obtained from Colorcon (Harleysville, PA). Hydrochloric acid, dichloromethane (DCM), methanol (MeOH), acetone and phosphate salts, maleic acid, sodium hydroxide were supplied by Fisher-Scientific (Pittsburg, PA). Biorelevant simulated gastric and intestinal fluids, including FaSSIF/FaSSGF, FeSSIF-V2 and FEDGAS were purchased from Biorelevant (London, UK).
Hanh Thuy Nguyen, Tu Van Duong, ynne S. Taylor, Enteric coating of tablets containing an amorphous solid dispersion of an enteric polymer and a weakly basic drug: a strategy to enhance in vitro release, International Journal of Pharmaceutics, 2023, 123139, ISSN 0378-5173, https://doi.org/10.1016/j.ijpharm.2023.123139.
Visit our new Webinar:
Solving capping challenges using mannitol as an excipient model
Get more information & register here:


