Mucoadhesive Buccal Film of Estradiol for Hormonal Replacement Therapy: Development and In-Vivo Performance Prediction
The age-related loss of circulating estrogen that occurs during the menopausal transition manifests itself through a variety of symptoms including vasomotor (hot flushes and night sweats), genito-urinary syndrome (vaginal dryness and urinary symptoms), sexual dysfunction, mood, and sleep disturbance that often last longer than a decade. Furthermore, reductions in estrogen level increase the risks of chronic complications such as osteoporosis, cardiovascular disease, and cognitive decline among others, thereby affecting the quality of life of women. Although oral estrogens are the most widely used therapy for menopausal symptoms, they suffer from poor bioavailability, and there are concerns over their safety, creating a significant concern to consumers. Mucoadhesive buccal films are an innovative dosage form that offers several advantages including avoidance of the first-pass metabolism, fast onset of action, and importantly, improved patient acceptance. In the current work, we developed mucoadhesive estradiol film for hormonal replacement therapy using film-forming polymers.
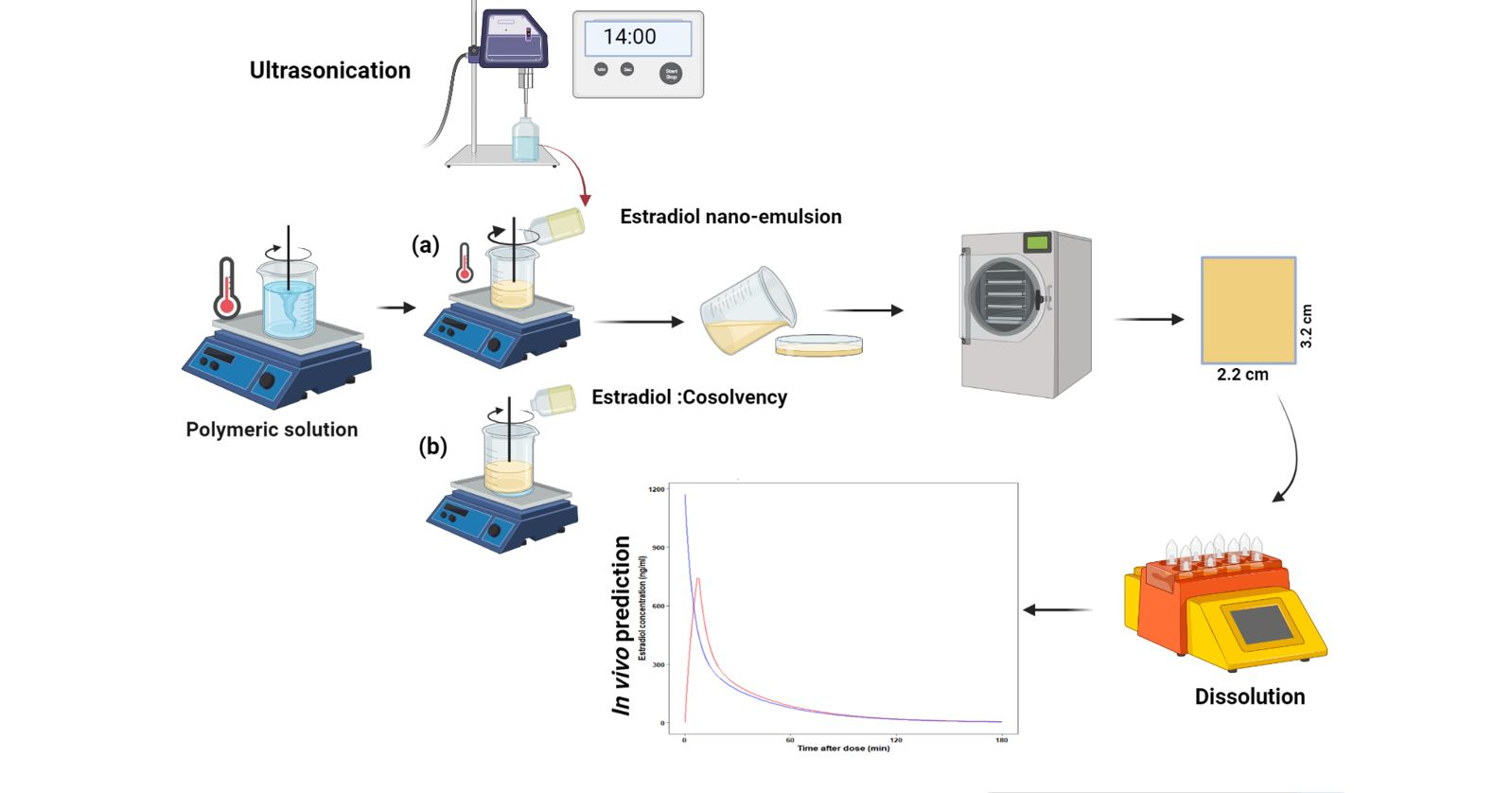
Two approaches, namely, co-solvency and nano-emulsion were evaluated to increase solubility and hence incorporate estradiol, a poorly water-soluble drug, into a formulation made from the hydrophilic polymer/s. The films were characterised for their mechanical and physicochemical properties. In-vitro release study showed that about 80% of the drug was released within 6 min from films prepared by the nano-emulsion approach, whereas it took about 10.5 min to get similar drug release from films prepared by the co-solvency approach. The ex-vivo permeation result indicates that about 15% of the drug permeated across the porcine buccal mucosa in the first 10 h from films prepared by the nano-emulsion approach, while permeation across porcine buccal mucosa was only observed at around 24 h from films prepared by the co-solvency method. The nano-emulsion films were evaluated for in vivo performance using a convolution technique using R software. The predicted Cmax and Tmax were found to be 740.74 ng mL−1 and 7 min, respectively, which were higher than previously reported in vivo concentration from oral tablets. The results demonstrated that mucoadhesive film of estradiol based on the nano-emulsion approach could be a promising platform for the delivery of estradiol through the buccal mucosa for the treatment of menopausal symptoms.
Abou this article: Abdella, S.; Afinjuomo, F.; Song, Y.; Upton, R.; Garg, S. Mucoadhesive Buccal Film of Estradiol for Hormonal Replacement Therapy: Development and In-Vivo Performance Prediction. Pharmaceutics 2022, 14, 542. https://doi.org/10.3390/pharmaceutics14030542
Materials
Different film-forming polymers were investigated either alone or in combination. Poly (vinyl alcohol) (15,000 Mw 80–89% hydroxylated), polyvinylpyrrolidone (PVP K-30 PI), citric acid, Tween® 80 (Polyoxyethylene Sorbitan Monooleate), Transcutol P oil, sucralose, and Poly (ethylene glycol) (PEG 400) were acquired from Sigma-Aldrich Merck (St. Louis, MO, USA); A model drug estradiol USP (E2) and xanthan gum were purchased from PCCA (Matraville, New South Wale, Australia). Hydroxypropyl methylcellulose (METHOCEL™ E50) was donated by Colorcon (Melbourne, Victoria, Australia). All chemicals were of pharmaceutical or analytic grade and used as received without any further modification or purification.

