Physiologically relevant model to establish the in vivo-in vitro correlation for etamsylate controlled release matrix tablets
Background
In vivo-in vitro correlation (IVIVC) are used during product development for optimization of drug formulations and can serve as a surrogate for bioequivalence studies.
Aim
This research was performed to investigate the in vitro dissolution and in vivo pharmacokinetics of different etamsylate immediate release (IR), and two controlled release (CR) matrix tablets with different dissolution rates; CRfast and CRslow. Also, to develop a simple physiological model to establish the IVIVC for etamsylate CR tablets.
Method
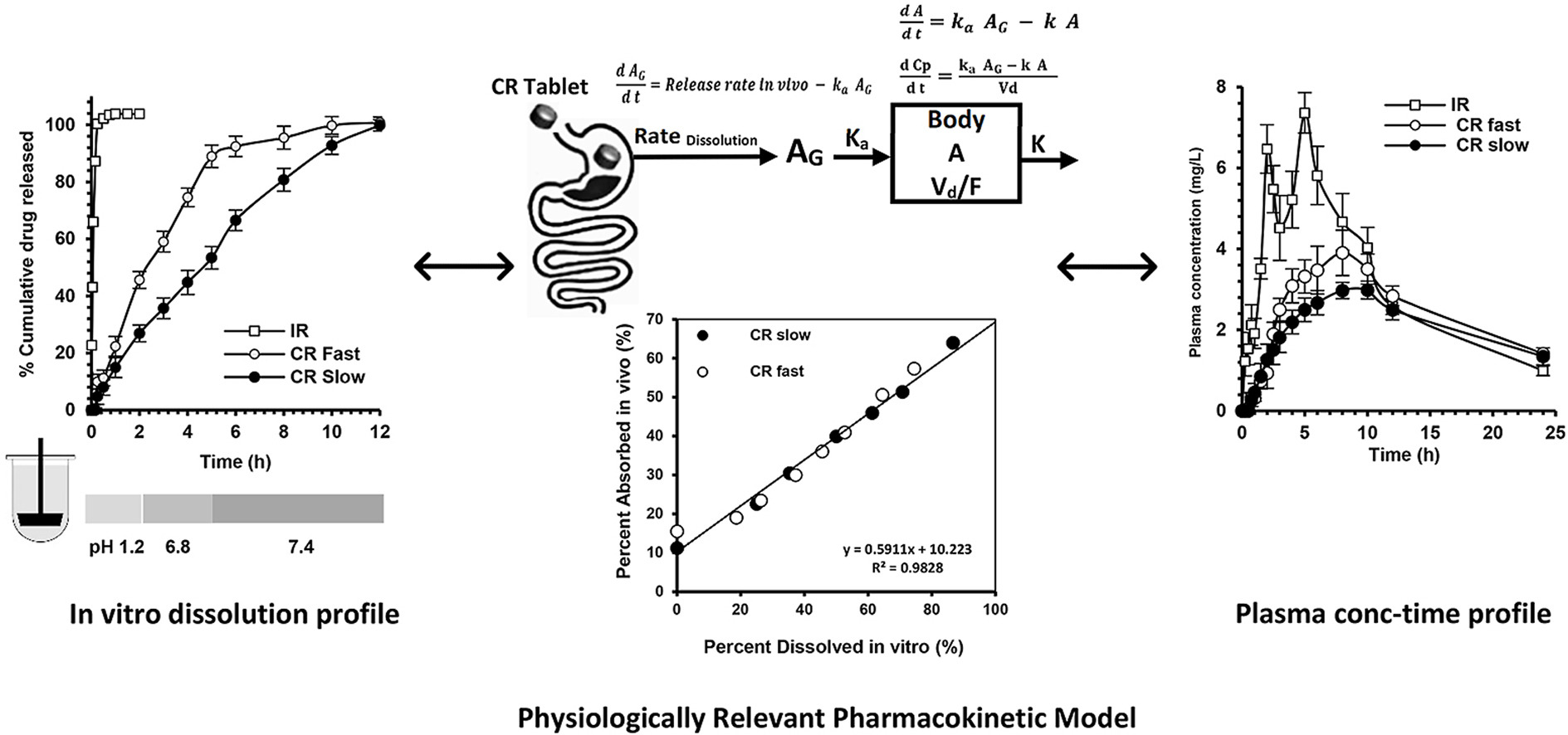
Three different formulations containing 500 mg etamsylate were developed; IR, CRfast, and CRslow tablets. The in vitro dissolution of the IR tablets was examined at different pHs, while the in vitro dissolution of the CR tablets was performed by the buffer transition method, where the pH of the dissolution medium was changed throughout the 12-h dissolution experiment. A group of eight healthy volunteers received one tablet of each formulation, in a three-way cross-over experimental design, with one-week wash-out period between treatments. The pharmacokinetic parameters of etamsylate after the three treatments were estimated. A model that utilized the in vitro dissolution data, the estimated pharmacokinetic parameters after the IR tablets was developed to establish the IVIVC for the CR tablets.
Results
The in vitro dissolution of the IR tablets showed a pH-dependent dissolution. The plasma concentration-time profile after administration of etamsylate IR tablet followed one-compartment model with first-order absorption and elimination. The maximum concentrations were 7.52 ± 2.1, 3.93 ± 0.96, and 3.13 ± 0.27 mg/L, and the time to achieve the maximum concentration was 5, 8, and 10 h, and the area under the curves were 80.2 ± 13.73, 69.4 ± 12.97, and 61.1 ± 0.44 mg-h/L for the IR, CRfast and CRslow tablets, respectively. There was a good agreement between the model-predicted concentration-time profile and the experimentally observed drug profile after administration of the two CR formulations. The model predicted Cmax and AUC were within 10% of the observed Cmax and AUC. Also, there was very good correlation between the time-scaled % in vivo absorption and the % in vitro dissolution for the two CR matrix tablets, indicating validity of the model.
Conclusion
The use of the buffer transition method was appropriate in predicting the in vivo dissolution of the developed CR matrix tablets. The developed model was valid in the establishment of the IVIVC for etamsylate CR tablets.
Article information: Mohsen A. Hedaya, Soha M. El-Masry, Sally A. Helmy, Physiologically relevant model to establish the in vivo-in vitro correlation for etamsylate controlled release matrix tablets, Journal of Drug Delivery Science and Technology, 2021. https://doi.org/10.1016/j.jddst.2021.102864.

