Comparative Evaluation of the Powder and Tableting Properties of Regular and Direct Compression Hypromellose from Different Vendors
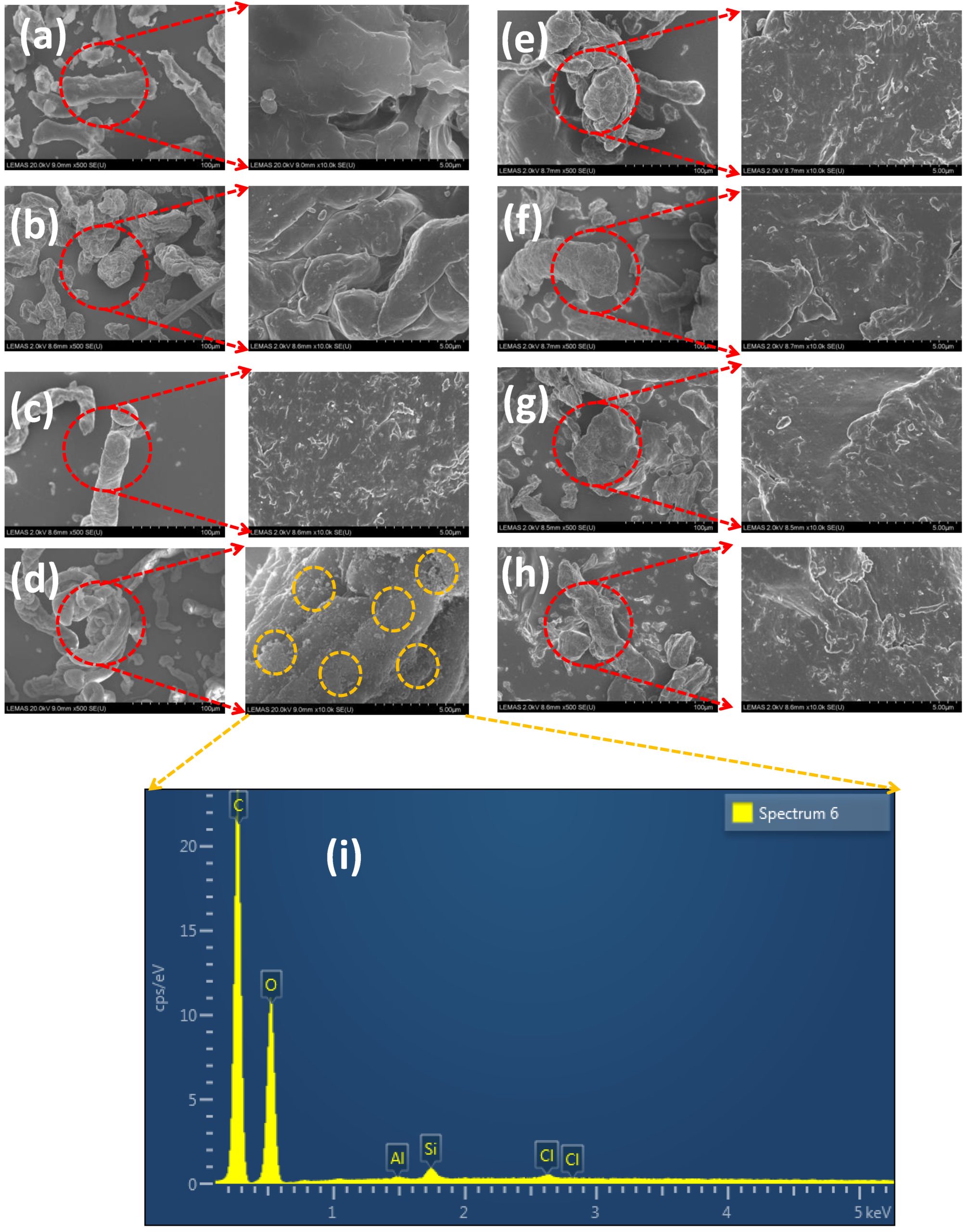
Hypromellose, a widely used polymer in the pharmaceutical industry, is available in several grades, depending on the percentage of substitution of the methoxyl and hydroxypropyl groups and molecular weight, and in various functional forms (e.g., suitable for direct compression tableting). These differences can affect their physicomechanical properties, and so this study aims to characterise the particle size and mechanical properties of HPMC K100M polymer grades from four different vendors. Eight polymers (CR and DC grades) were analysed using scanning electron microscopy (SEM) and light microscopy automated image analysis particle characterisation to examine the powder’s particle morphology and particle size distribution. Bulk density, tapped density, and true density of the materials were also analysed. Flow was determined using a shear cell tester. Flat-faced polymer compacts were made at five different compression forces and the mechanical properties of the compacts were evaluated to give an indication of the powder’s capacity to form a tablet with desirable strength under specific pressures. The results indicated that the CR grades of the polymers displayed a smaller particle size and better mechanical properties compared to the DC grade HPMC K100M polymers. The DC grades, however, had better flow properties than their CR counterparts. The results also suggested some similarities and differences between some of the polymers from the different vendors despite the similarity in substitution level, reminding the user that care and consideration should be given when substitution is required.
1. Introduction
2.1. Materials
Download the full study as PDF here: Comparative Evaluation of the Powder and Tableting Properties of Regular and Direct Compression Hypromellose from Different Vendors
or read it here
Mawla, N.; Alshafiee, M.; Gamble, J.; Tobyn, M.; Liu, L.; Walton, K.; Conway, B.R.; Timmins, P.; Asare-Addo, K. Comparative Evaluation of the Powder and Tableting Properties of Regular and Direct Compression Hypromellose from Different Vendors. Pharmaceutics 2023, 15, 2154.
https://doi.org/10.3390/pharmaceutics15082154

