Modeling the Impact of Excipients Selection on Nitrosamine Formation towards Risk Mitigation
Abstract
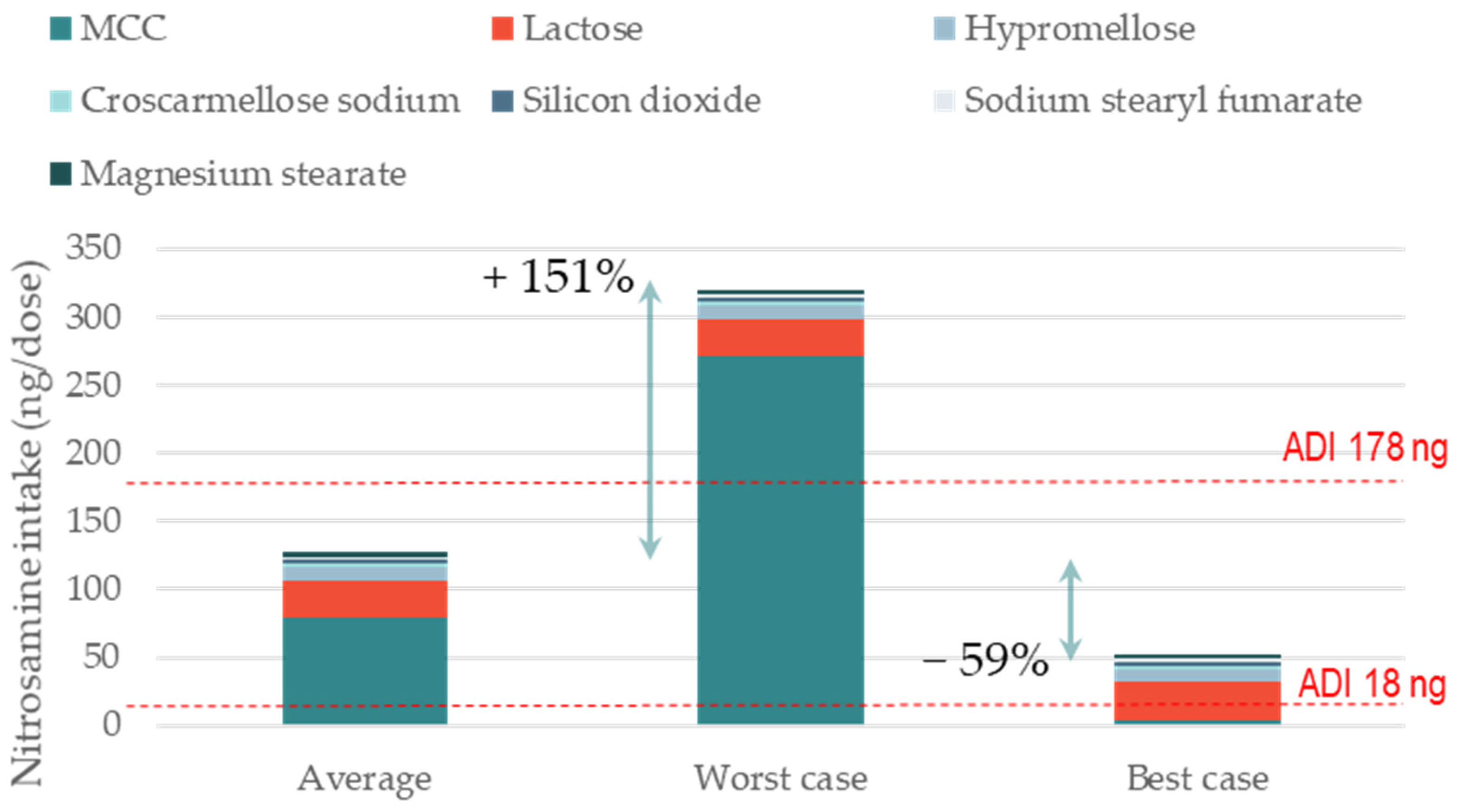
Risk control for nitrosamine impurities in drug products is currently a major challenge in the industry. Nitrosamines can form during drug product manufacturing and storage through the reaction of nitrites with amine-containing APIs or impurities. The level of nitrites in excipients and the rate of reaction often control the build-up of nitrosamine. Although the variability in nitrite levels across excipient types and suppliers is well recognized, the impact of excipient selection on the level of nitrosamine formed has not been systematically studied. This gap of knowledge is addressed in the current work. We present theoretical case studies of formulations where microcrystalline cellulose (MCC), or lactose supplier, or superdisintegrant type are changed in pursuit of lower levels of nitrite. The impact of the average, maximum, and minimum levels of nitrites in each excipient on nitrosamine formation in the dosage form is calculated. The input data for this calculation are the formulation composition, nitrosamine molecular weight (MW), percentage of conversion, and nitrite levels per excipient. The percentage of conversion (based on the formulation and manufacturing variables) and nitrite levels were taken from the recent literature. We show that changing the supplier of a single excipient, or of the three most critical excipients, can reduce nitrosamine formation by up to −59% and −89%, respectively. We also show that high-risk formulations, e.g., high MW nitrosamines, high dosage weights, and high percentages of conversion (e.g., wet granulation), can often be de-risked below regulatory acceptable daily intake via careful excipient selection. Finally, we provide an open-access tool that enables users to calculate the theoretical formation of nitrosamines in their specific formulations. This calculation template can be used for (i) the preliminary screening of the risk of nitrosamine formation in drug products and (ii) the preliminary assessment of the impact of excipient selection for risk mitigation.
Introduction
Nitrosamines are recognized to be probable or possible human cancerogenic substances and have been found in many pharmaceutical products [1,2]. Regulatory agencies worldwide have recently prompted marketing authorization holders (MAHs) to take action in relation to this health concern [3]. Both the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have issued guidance indicating the steps that MAHs should follow to evaluate the risk of nitrosamines, test for their presence, and eventually implement changes in pursuit of the reduction in nitrosamines in drug products. About 15% to 40% of all active pharmaceutical ingredients (APIs) are at risk of nitrosamine formation, and this challenge is shared across different compounds and pharmacological classes [4].
Nitrosamines are not directly cancerogenic, but require metabolic activation by certain enzymes of the P450 cytochrome family. Nitrosamines can be converted into alkylating agents (typically alkyldiazonium ions) that can interact with DNA [5]. Both metabolism and alkylation steps are covalent reactions that are typically influenced by steric and electronic interactions. Hence, the specific chemical features of a given nitrosamine can either decrease or increase their potency. A second aspect that could influence the cancerogenic potential of a nitrosamine is related to biological and pharmacokinetic complexities. Factors like absorption, distribution, clearance, and metabolism within physiological conditions could all influence the potency of a given nitrosamine. For example, aromatic N-nitrosamine can be subjected to Fischer–Hepp rearrangement under acidic conditions to form para-nitrosoarylamines. For a deeper understanding of the potency/mutagenicity of various nitrosamines, readers are referred to articles in the recent literature [4,6,7,8,9]. The topic of nitrosamine potency goes beyond the focus of this work. Here, we show the impact of excipient selection on nitrosamines formed during processing and tablet storage, with the objective of mitigating the risk to exceed acceptable daily intakes (ADIs) proposed by regulatory agencies.
Understanding the pathway to nitrosamine formation is key to developing appropriate mitigation strategies for nitrosamine reduction. Nitrosamines typically form through the reaction of secondary amines with nitrosating agents. Extensive research over the past few years has shown that secondary amines inherently present in APIs, present as impurities, or formed as degradation products of APIs can interact with nitrosating agents present in the formulation [10,11]. Nitrites that are present as impurities in excipients are the main source of nitrosating agents. The rate-limiting reagent in the formation of nitrosamines is typically the nitrite present at trace levels rather than the much more abundant secondary amine.
The formation of nitrosamines from nitrites in a drug product is often determined by two factors:
The level of nitrites in the formulation;
The percentage of conversion of nitrites into nitrosamines.
Risk assessments of drug products are usually conducted considering the worst-case scenario (i.e., the theoretical maximum levels) of both the nitrite levels and the extent of conversion (i.e., 100%) [12]. The risk is finally determined by comparing the calculated levels against the ADI for the nitrosamine of interest [3]. As the understanding of this topic is rapidly evolving, it is becoming apparent that the calculated nitrosamine content based on both the worst-case scenarios of nitrite levels in excipients and 100% conversion likely results in a largely overestimated risk compared to realistic conditions.
Moser et al. recently determined that the 100% conversion of nitrite into nitrosamines in the solid state is far from accurate [13]. In all cases studied, the level of N-nitrosamine formation in solid dosage forms plateaued at a level much lower than 100%. A maximum conversion of approximately 38% was measured in tablet dosage forms with the most favorable conditions for nitrosamine formation during stability tests. In all cases, one could argue that this worst-case scenario of 38% conversion of nitrites into nitrosamines is largely approximative. This is because the nitrite content in the formulations was based on the average levels reported in the literature, whereas the formation of nitrosamines was measured experimentally. Given the extremely high supplier-to-supplier variability of nitrites in excipients [12], the estimated nitrite level used in the article by Moser et al. might be vastly different from the actual nitrite content. Hence, the calculation of the conversion of nitrites into nitrosamines could, in turn, be approximative. Interestingly, Moser et al. also tested tablets spiked with larger and known quantities of nitrites and then measured the nitrosamine formation. The resulting calculation of the percentage of conversion was more realistic in this case, given that both the nitrite and nitrosamine contents were known experimentally. The highest percentage of conversion measured in the spiked formulations was 29%. It was also proven that the most favorable conditions for maximum conversion were (1) large excesses of secondary amine API; (2) API in the salt form; (3) wet granulation; and (4) storage in high-humidity conditions [13].
Moser et al. established, for the first time, a point of reference for the expected percentage of the conversion of nitrites into nitrosamines in tablets. In addition to the percentage of conversion, the second important factor determining nitrosamine content in a tablet is the nitrite content of excipients used. In a pioneering work, Schlingerman et al. recently showed a reduction in nitrosamine formation in a drug product via the selection of the excipient supplier providing the lowest level of nitrites [14]. However, so far, the impact of the selection of excipients with different levels of nitrite on the formation of nitrosamines has not been systematically studied [10,14]. As regulatory bodies indicate supplier qualification (e.g., a change of excipient supplier) and formulation design (e.g., a change of excipient type) as the main mitigation strategies to reduce nitrosamines [15], it is key to understand the extent that these strategies can reduce the risk of nitrosamine formation. Such an evaluation needs to take into account the realistic values of both nitrite levels in excipients and the percentage of conversion into nitrosamines (not 100%).
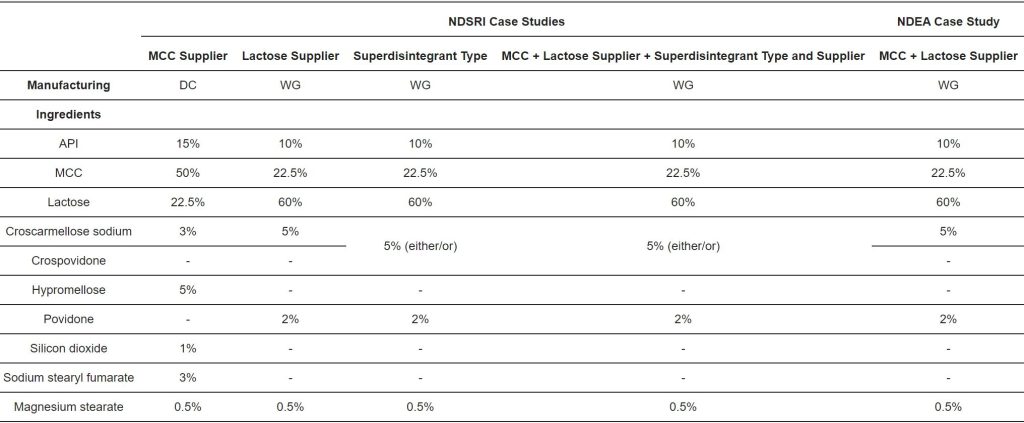
In this study, we calculate the theoretical nitrosamine content for model formulations using realistic percentages of conversion and the average content of nitrites, as indicated in the literature. Then, we calculate to what extent the nitrosamine content can change by replacing either the supplier of an excipient or the type of excipient in the formulation. The impact of specific variables such as the tablet dose weight, nitrosamine MW, and process of manufacturing (dry process vs. wet granulation) on nitrosamine formation and the consequent risk of exceeding regulatory thresholds is also evaluated. Finally, a calculation tool is provided whereby the user can directly calculate the contribution of each excipient on the theoretical formation of nitrosamine in a formulation. The input parameters for the calculation are the tablet weight and composition and nitrosamine MW. The percentages of conversion into nitrosamines, as well as the levels of nitrites in excipients, are provided based on the scientific literature [12,13].
The simulation presented here showcases to what extent the change of a single ingredient in a formulation can impact nitrosamines levels. Moreover, this article provides a preliminary framework for the preparation of nitrosamine risk assessments for drug products, which reflects the realistic values of both the nitrite content and the percentage of conversion.
Download the full article as PDF here: Modeling the Impact of Excipients Selection on Nitrosamine Formation towards Risk Mitigation
or read it here
Berardi, A.; Jaspers, M.; Dickhoff, B.H.J. Modeling the Impact of Excipients Selection on Nitrosamine Formation towards Risk Mitigation. Pharmaceutics 2023, 15, 2015. https://doi.org/10.3390/pharmaceutics15082015

