Characterizing a design space for a twin-screw wet granulation process: A case study of extended-release tablets
Abstract
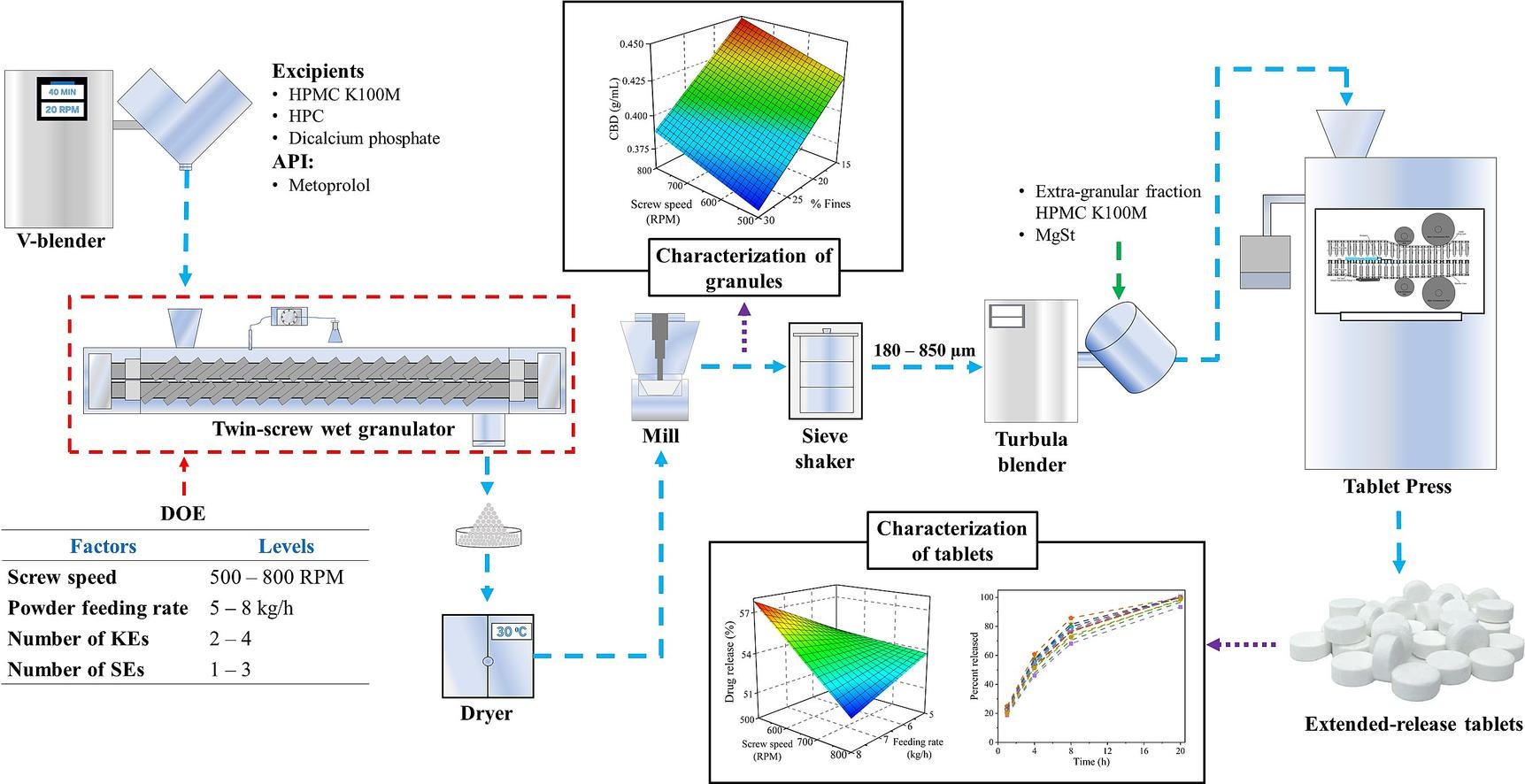
Twin-screw wet granulation is an emerging continuous manufacturing technology for solid oral dosage forms. This technology has been successfully employed for the commercial manufacture of immediate-released tablets. However, the higher polymer content in extended-release (ER) formulations may present challenges in developing and operating within a desired design space. The work described here used a systematic approach for defining the optimum design space by understanding the effects of the screw design, operating parameters, and their interactions on the critical characteristics of granules and ER tablets. The impacts of screw speed, powder feeding rate, and the number of kneading (KEs) and sizing elements on granules and tablets characteristics were investigated by employing a definitive screening design. A semi-mechanistic model was used to calculate the residence time distribution parameters and validated using the tracers.
The results showed that an increase in screw speed decreased the mean residence time of the material within the barrel, while an increase in the powder feeding rate or number of KEs did the opposite and increased the barrel residence time. Screw design and operating parameters affected the flow and bulk characteristics of granules. The screw speed was the most significant factor impacting the tablet’s breaking strength. The dissolution profiles revealed that granule characteristics mainly influenced the early phase of drug release. This study demonstrated that a simultaneous optimization of both operating and screw design parameters was beneficial in producing ER granules and tablets of desired performance characteristics while mitigating any failure risks, such as swelling during processing.
Introduction
Twin-screw wet granulation (TSWG) is an emerging continuous manufacturing technology for solid oral dosage forms. The TSWG technology has gained considerable interest in the pharmaceutical industry due to short processing times, design flexibility, intermeshing kneading process, and the potential for high drug load formulation (Dhenge et al., 2010, Meier et al., 2015, Vercruysse et al., 2015, Zidan et al., 2022). Wet granulation is a particle size enlargement process which forms larger multi-unit agglomerates from a fine powder blend with the help of a liquid binder solution. Three granulation-rate processes govern the formation of granules (Hapgood et al., 2007). First, wetting and nucleation, where the binder solution is added and even distributed in the dry feeding powder to form the nuclei. Second, consolidation and growth, in which the nuclei grow to form wet granules or agglomerates by collisions within the granulator. Third, breakage and attrition of wet agglomerates into final granules by impact, shear or compaction in the granulator. These rate processes are essential to determine the final properties of the granules.
TSWG process may produces granules of acceptable flow and compaction properties, minimizes the dust while increasing the particle size and bulk density of materials processed (Nandi et al., 2021). TSWG has been shown to be sensitive to formulation attributes such as drug load and concentration and distribution of excipients (Hiremath et al., 2019, Matsunami et al., 2023, Meier et al., 2015); operating parameters such as powder feeding rate, screw speed, and liquid-to-solid (L/S) ratio (Dhenge et al., 2011, Dhenge et al., 2010, Lute et al., 2018); and screw design parameters such as the number of conveying elements, number of kneading zones, number of kneading elements (KEs) per kneading zone, and sizing elements (SEs) at the end of the screw (Djuric and Kleinebudde, 2008, Vercruysse et al., 2012, Zidan et al., 2022). Understanding the impact of these factors (and their interactions) on processability, optimization of the design space, and integrated process control strategies that enable real-time product quality monitoring and control is essential for quality drug manufacturing by TSWG.
Powder feeding rate is an operating parameter that may increase the mass hold-up within the TSWG barrel, the compressive and shear stresses exerted (Lute et al., 2018), and the sphericity and strength of granules (Dhenge et al., 2011, Dhenge et al., 2010, Djuric et al., 2009, Lee et al., 2012). The fraction of fines (<150 µm) may increase without significantly affecting the yield (between 150 and 1400 µm) and agglomerates (>1400 µm) of the granules at higher powder feeding rates (Vercruysse et al., 2012). The screw speed is another important operating parameter in a TSWG process. The effect of screw speed on granule size depends on the characteristics of the binder liquid (e.g., composition, viscosity, surface tension) and binder liquid feed rate (Keleb et al., 2004). Keleb and coworkers observed an increase in granule size at high screw speed only when polyvinylpyrrolidone solution was used instead of water as a binder liquid. Further studies using water as a binder liquid demonstrated a non-significant effect of screw speed on granule size (Dhenge et al., 2010, Djuric et al., 2009).
The screw design parameters also have a significant effect on the properties of the granules. The number and distribution of conveying elements, KEs, and SEs along the screws affect the mechanism of granulation and granules properties. KEs facilitate the binder liquid distribution and increase the shear and compressive forces acting on the granules (Kotamarthy and Ramachandran, 2021, Vercruysse et al., 2012). A higher number of KEs per kneading zone generally results in denser granules, smaller fraction of fine granules, larger fraction of oversized granules, and a reduction in disintegration time (Djuric and Kleinebudde, 2008, Vercruysse et al., 2012). The number of KEs also impacts the morphology of granules. An increase in the elongated and irregular form of granules was observed at a higher number of KEs (El Hagrasy and Litster, 2013, Thompson and Sun, 2010, Zidan et al., 2022). SEs at the end of the screws control the particle size by adding a wet milling zone that promotes the breaking of the oversized granules (Vercruysse et al., 2015).
Residence time distribution (RTD) parameters such as mean residence time (MRT) and normalized variance are also influenced by formulation attributes, and screw design and operating parameters. RTD parameters are essential for understanding the dynamics of material flow in a TSWG process. A longer residence time may lead to more uniform wetting, mixing, and consolidation of particles (Karttunen et al., 2019), but a longer residence time may also lead to polymer swelling in extended-release (ER) formulations of high polymer content. Different empirical and statistical models have been developed to predict the MRT and 2 in TSWG processes (Ismail et al., 2019, Kumar et al., 2014, Kumar et al., 2015, Poulesquen et al., 2003). Although these models are a valuable tool for an initial approximation of the TSWG’s dynamics, they must be re-validated for different equipment. Muddu and coworkers proposed a semi-mechanistic model that may be extended to the TSWG process of different scales, screw design, and operating parameters, yielding lower prediction errors for a different set of experimental data (Muddu et al., 2021).
The studies mentioned above demonstrate the flexibility of the twin-screw wet granulation process to produce granules of desired characteristics. It may be important to note that these previous studies were performed for processing immediate-release solid oral formulations. There is a paucity of information on the effect of operating parameters and screw design parameters for processing ER formulations using TSWG. Understanding these effects is essential for switching from batch to continuous a granulation process for ER formulations (Kim et al., 2017, Kotamarthy et al., 2022). The higher content of release-controlling polymer in the ER formulations is expected to limit the design space for optimizing screw design and operating conditions (Vanhoorne et al., 2016). Swelling of these polymers and their premature consolidation may increase the torque and lead to the failure of a TSWG process (Kotamarthy et al., 2022). An inadequate L/S ratio, excess of shear stress, and higher MRT in the TSWG barrel may lead to swelling failure in a TSWG process. Our previous work highlighted the impact of screw design parameters on the attributes of granules and ER tablets produced using the TSWG process (Zidan et al., 2022). Our study demonstrated that the number of KEs, the staggering angle of KEs, and the number of SEs significantly affected the particle size distribution (PSD), the bulk and flow properties of the granules, and the breaking strength and dissolution rate of the ER tablets. The distance between kneading zones did not significantly affect granules and tablet characteristics. Nevertheless, the influence of operating parameters and their interactions with the critical screw design parameters on the properties of granules and ER tablets has yet to be investigated.
The objective of the current study was to characterize the design space for a twin-screw wet granulation processing an ER formulation of metoprolol succinate (a model API). A definitive screening design was used to understand the effects of number of KEs and SEs, screw speed, and powder feeding rate on RTD parameters and characteristics of granules and ER tablets. The response parameters for flow dynamics within the TSWG barrel included MRT and normalized variance, while the response parameters of granules attributes included PSD, conditioned bulk density (CBD), compressibility (CPS), cohesion, flow factor, permeability, basic flow energy (BFE), and specific energy. The response parameters for ER tablets included breaking strength and dissolution characteristics.
Read more here
Materials
Metoprolol succinate (UniChem Laboratories Ltd, Mumbai, India) was used as a model active pharmaceutical ingredient (API). The formulation included hydroxypropyl methylcellulose (Methocel HPMC K100M Premium CR, Dow Chemical Company, Midland, Michigan, USA), calcium hydrogen phosphate dihydrate (DCP dihydrate, EMCOMPRESS®, JRS Pharma GMBH& Co., Patterson, New York, USA), hydroxypropyl cellulose (HPC 100 K, MilliporeSigma, St. Louis, Missouri, USA), magnesium stearate.
Nobel O. Sierra-Vega, Fahd M. Alsharif, Thomas O’Connor, Muhammad Ashraf, Ahmed Zidan, Characterizing a design space for a twin-screw wet granulation process: A case study of extended-release tablets, International Journal of Pharmaceutics, Volume 650, 2024, 123681, ISSN 0378-5173, https://doi.org/10.1016/j.ijpharm.2023.123681.

