Formulation development and evaluation of fluvastatin loaded transethosomes: Characterization, stability, in vitro dermal penetration, cytotoxicity and antipsoriatic activity studies
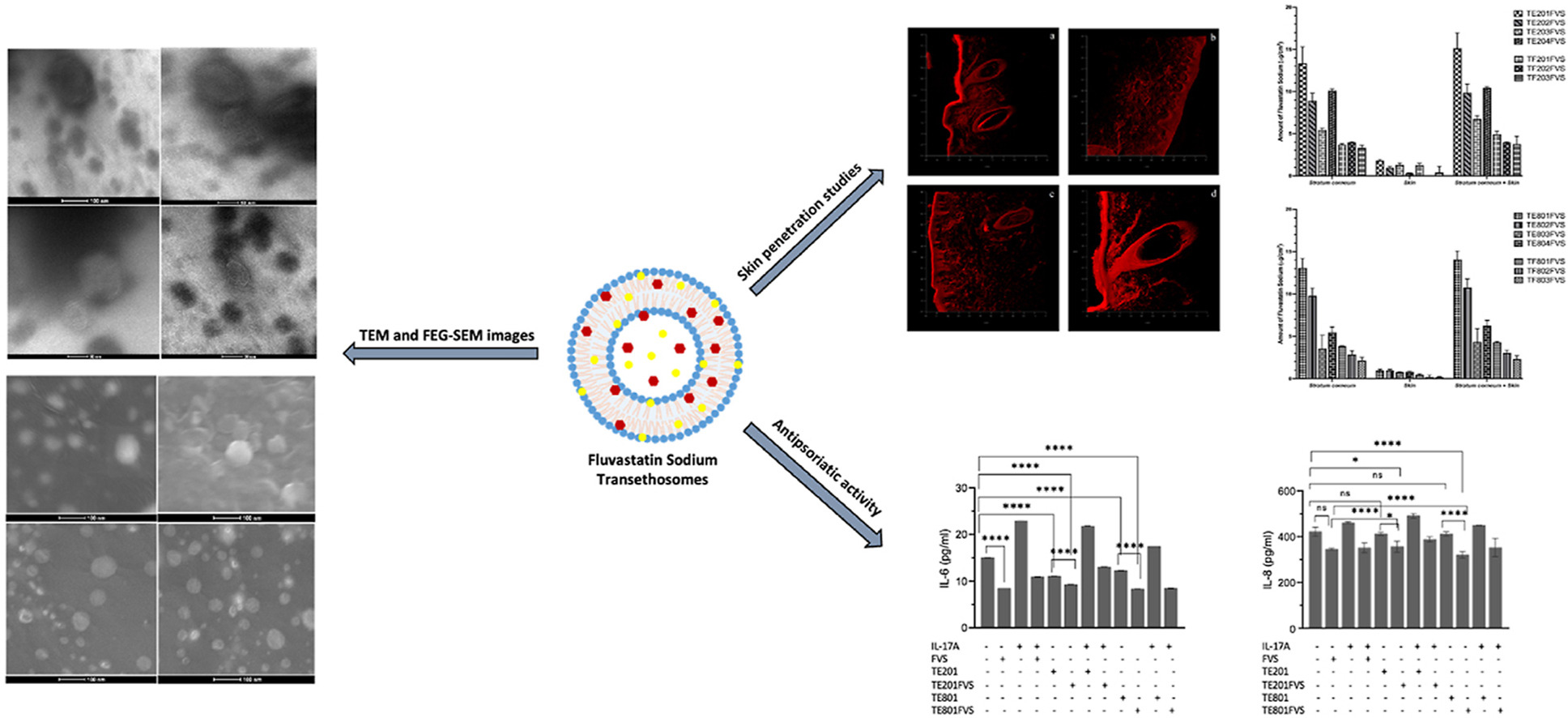
Psoriasis, a common immune-mediated chronic inflammatory disease, may arise from genetic, epigenetic, and immune system factors. Current medications focus on symptom relief, presenting a challenge in discovering new compounds or methods for safe and effective psoriasis management while ensuring patient adherence. Statin drugs like fluvastatin, initially for dyslipidemia, were considered beneficial for treating psoriasis. Thus the objective of this study was to formulate novel topical transethosomes as drug delivery systems for dermal administration of fluvastatin sodium (fluvastatin) in psoriasis treatment. The thin film hydration method was exploited to prepare fluvastatin loaded transethosomes consisted of Phospholipon 90 as the phospholipid, along with Tween 20 or Tween 80 as the edge activators. The physicochemical properties of the transethosomes, including particle size and size distribution, zeta potential, encapsulation efficiency, pH, and conductivity, were evaluated. Attenuated Total Reflectance Fourier Transformed Infrared (ATR-FTIR) analysis was conducted to determine the structural characteristics, while Transmission Electron Microscopy (TEM) and Scanning Electron Microscopy (SEM) analyses were employed to examine the morphological properties. Additionally, the stability of the transethosomes was monitored. Transfersome-type delivery systems were also developed for comparison with the transethosome formulations. The in vitro skin penetration and deposition behavior of fluvastatin transethosomes were assessed using sequential tape stripping, ATR-FTIR Spectroscopy, and Confocal Laser Scanning Microscopy (CLSM) and compared to fluvastatin-loaded transfersomes. Furthermore, in vitro cell culture studies were conducted to evaluate the cytotoxicity and antipsoriatic activities of the fluvastatin-loaded transethosomal carrier systems that exhibited the highest dermal accumulation. The results suggest that fluvastatin-loaded transethosomes inhibit the IL6 and IL8 production in HaCaT cells compared to free fluvastatin. Overall, the aim of this research is to develop innovative, skin-compatible, and stable drug delivery systems that improve the effectiveness of psoriasis treatment. This would be achieved by targeting the active substance, fluvastatin, which is believed to have potential antipsoriatic effects, specifically to the affected area of the skin.
Materials
Fluvastatin sodium was kindly provided by Aurobindo Pharma (Hyderabad, India). Phospholipon 90G (Purified phosphatidylcholine from soybean lecithin, P90G) was a kind gift of Lipoid GmbH (Ludwigshafen, Germany). Polyoxyethylene sorbitan monolaurate (Tween 20), Polyoxyethylene sorbitan monooleate (Tween 80), and Nile Red were purchased from Sigma-Aldrich Chemie Gmbh (Steinheim, Germany). The human immortalized keratinocyte (HaCaT; T0020001) cell line was purchased from AddexBio (USA). Dulbecco’s
Read more
Aslı Gürbüz Yurtsever, Ayşegül Ekmekcioglu, Meltem Muftuoglu, Sevgi Güngör, Meryem Sedef Erdal, Formulation development and evaluation of fluvastatin loaded transethosomes: Characterization, stability, in vitro dermal penetration, cytotoxicity and antipsoriatic activity studies, Journal of Drug Delivery Science and Technology, 2023, 105234, ISSN 1773-2247,
https://doi.org/10.1016/j.jddst.2023.105234.

