Formulating Drug Products for Optimized Absorption: Elucidating Amorphous Solid Dispersions
CDER scientists are seeking ways to improve the bioavailability of drugs that on their own do not dissolve well in water. Recent CDER research explores the potential for using amorphous solid dispersions to formulate generic drug products that may include ingredients that are poorly water-soluble.
Introduction
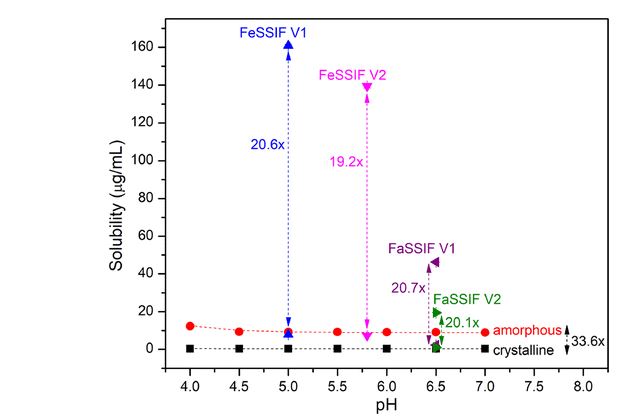
Amorphous solid dispersions (ASDs) are a popular mechanism for enhancing the solubility and bioavailability of drugs that are poorly soluble in water. Important drugs that treat cancer, cystic fibrosis, and organ transplant rejection, to name a few, use ASDs in their formulation to help overcome the solubility limitations of drugs when they are administered orally. Recent research conducted by FDA’s Office of Generic Drugs in the Center for Drug Evaluation and Research explored the mechanistic understanding and prediction of in vivo performance of ASD drug products. Findings from the research create a valuable foundation for understanding the impact of biologically relevant media on solution phase behavior of poorly soluble drugs which can assist in the development of generic drug products that will use ASDs. The research results are also helpful for the regulatory assessment and evaluation of the bioinequivalence risk of the test drug products compared to the reference drug products.
Continue to read the complete FDA article
How does this research improve generic drug development and support their approval?
This research explores the current mechanistic understanding and prediction of in vivo performance of amorphous solid dispersions drug products for test and reference drug. This research may be valuable for the development of generic drug products used to treat diseases such as cancer, cystic fibrosis, and heart, liver, and lung transplant rejection. This better understanding will also be critical to guide the regulatory assessment and evaluation for test drug products regarding the risk of not being bioequivalent compared to their reference standard.
Source: FDA

